Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation
- PMID: 20808845
- PMCID: PMC2924363
- DOI: 10.1371/journal.ppat.1001056
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation
Abstract
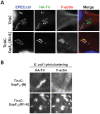
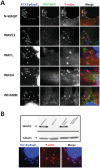
Upon infection of mammalian cells, enterohemorrhagic E. coli (EHEC) O157:H7 utilizes a type III secretion system to translocate the effectors Tir and EspF(U) (aka TccP) that trigger the formation of F-actin-rich 'pedestals' beneath bound bacteria. EspF(U) is localized to the plasma membrane by Tir and binds the nucleation-promoting factor N-WASP, which in turn activates the Arp2/3 actin assembly complex. Although N-WASP has been shown to be required for EHEC pedestal formation, the precise steps in the process that it influences have not been determined. We found that N-WASP and actin assembly promote EHEC-mediated translocation of Tir and EspF(U) into mammalian host cells. When we utilized the related pathogen enteropathogenic E. coli to enhance type III translocation of EHEC Tir and EspF(U), we found surprisingly that actin pedestals were generated on N-WASP-deficient cells. Similar to pedestal formation on wild type cells, Tir and EspF(U) were the only bacterial effectors required for pedestal formation, and the EspF(U) sequences required to interact with N-WASP were found to also be essential to stimulate this alternate actin assembly pathway. In the absence of N-WASP, the Arp2/3 complex was both recruited to sites of bacterial attachment and required for actin assembly. Our results indicate that actin assembly facilitates type III translocation, and reveal that EspF(U), presumably by recruiting an alternate host factor that can signal to the Arp2/3 complex, exhibits remarkable versatility in its strategies for stimulating actin polymerization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
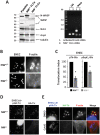
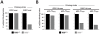
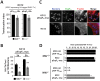
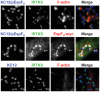



Similar articles
-
Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly.PLoS Pathog. 2008 Oct;4(10):e1000191. doi: 10.1371/journal.ppat.1000191. Epub 2008 Oct 31. PLoS Pathog. 2008. PMID: 18974829 Free PMC article.
-
Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6754-9. doi: 10.1073/pnas.0809131106. Epub 2009 Apr 6. Proc Natl Acad Sci U S A. 2009. PMID: 19366662 Free PMC article.
-
EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly.Dev Cell. 2004 Aug;7(2):217-28. doi: 10.1016/j.devcel.2004.07.004. Dev Cell. 2004. PMID: 15296718
-
Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal.Cell Microbiol. 2008 Mar;10(3):549-56. doi: 10.1111/j.1462-5822.2007.01103.x. Epub 2007 Dec 4. Cell Microbiol. 2008. PMID: 18053003 Review.
-
Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly.FEBS J. 2010 Jun;277(11):2390-402. doi: 10.1111/j.1742-4658.2010.07653.x. Epub 2010 Apr 27. FEBS J. 2010. PMID: 20477869 Review.
Cited by
-
The Chlamydia trachomatis secreted effector TmeA hijacks the N-WASP-ARP2/3 actin remodeling axis to facilitate cellular invasion.PLoS Pathog. 2020 Sep 18;16(9):e1008878. doi: 10.1371/journal.ppat.1008878. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32946535 Free PMC article.
-
Human endothelial cells internalize Candida parapsilosis via N-WASP-mediated endocytosis.Infect Immun. 2013 Aug;81(8):2777-87. doi: 10.1128/IAI.00535-13. Epub 2013 May 20. Infect Immun. 2013. PMID: 23690407 Free PMC article.
-
Shiga toxin in enterohemorrhagic E.coli: regulation and novel anti-virulence strategies.Front Cell Infect Microbiol. 2012 Jun 7;2:81. doi: 10.3389/fcimb.2012.00081. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919672 Free PMC article. Review.
-
Chlamydia trachomatis TmeA promotes pedestal-like structure formation through N-WASP and TOCA-1 interactions.mSphere. 2025 May 27;10(5):e0010125. doi: 10.1128/msphere.00101-25. Epub 2025 Apr 15. mSphere. 2025. PMID: 40231845 Free PMC article.
-
Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector.Cell Adh Migr. 2014;8(4):404-17. doi: 10.4161/19336918.2014.969993. Cell Adh Migr. 2014. PMID: 25482634 Free PMC article.
References
-
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. - PubMed
-
- Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48:95–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

