Immune modulation with sulfasalazine attenuates immunopathogenesis but enhances macrophage-mediated fungal clearance during Pneumocystis pneumonia
- PMID: 20808846
- PMCID: PMC2924364
- DOI: 10.1371/journal.ppat.1001058
Immune modulation with sulfasalazine attenuates immunopathogenesis but enhances macrophage-mediated fungal clearance during Pneumocystis pneumonia
Abstract
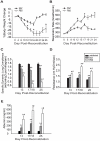
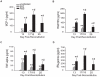
Although T cells are critical for host defense against respiratory fungal infections, they also contribute to the immunopathogenesis of Pneumocystis pneumonia (PcP). However, the precise downstream effector mechanisms by which T cells mediate these diverse processes are undefined. In the current study the effects of immune modulation with sulfasalazine were evaluated in a mouse model of PcP-related Immune Reconstitution Inflammatory Syndrome (PcP-IRIS). Recovery of T cell-mediated immunity in Pneumocystis-infected immunodeficient mice restored host defense, but also initiated the marked pulmonary inflammation and severe pulmonary function deficits characteristic of IRIS. Sulfasalazine produced a profound attenuation of IRIS, with the unexpected consequence of accelerated fungal clearance. To determine whether macrophage phagocytosis is an effector mechanism of T cell-mediated Pneumocystis clearance and whether sulfasalazine enhances clearance by altering alveolar macrophage phagocytic activity, a novel multispectral imaging flow cytometer-based method was developed to quantify the phagocytosis of Pneumocystis in vivo. Following immune reconstitution, alveolar macrophages from PcP-IRIS mice exhibited a dramatic increase in their ability to actively phagocytose Pneumocystis. Increased phagocytosis correlated temporally with fungal clearance, and required the presence of CD4(+) T cells. Sulfasalazine accelerated the onset of the CD4(+) T cell-dependent alveolar macrophage phagocytic response in PcP-IRIS mice, resulting in enhanced fungal clearance. Furthermore, sulfasalazine promoted a TH2-polarized cytokine environment in the lung, and sulfasalazine-enhanced phagocytosis of Pneumocystis was associated with an alternatively activated alveolar macrophage phenotype. These results provide evidence that macrophage phagocytosis is an important in vivo effector mechanism for T cell-mediated Pneumocystis clearance, and that macrophage phenotype can be altered to enhance phagocytosis without exacerbating inflammation. Immune modulation can diminish pulmonary inflammation while preserving host defense, and has therapeutic potential for the treatment of PcP-related immunopathogenesis.
Conflict of interest statement
Dr. George is an employee of Amnis Corporation, producers of the ImageStream imaging flow cytometer.
Figures

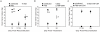


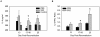

References
-
- Santos J, Palacios R, Ruiz J, Gonzalez M, Marquez M. Study of patients diagnosed with advanced HIV in the HAART era—OMEGA Cohort. Int J STD AIDS. 2005;16:252–255. - PubMed
-
- Forrest DM, Seminari E, Hogg RS, Yip B, Raboud J, et al. The incidence and spectrum of AIDS-defining illnesses in persons treated with antiretroviral drugs. Clin Infect Dis. 1998;27:1379–1385. - PubMed
-
- Staikowsky F, Lafon B, Guidet B, Denis M, Mayaud C, et al. Mechanical ventilation for Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Is the prognosis really improved? Chest. 1993;104:756–762. - PubMed
-
- Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med. 1995;155:2436–2441. - PubMed
-
- Kaur N, Mahl TC. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn's disease. Dig Dis Sci. 2004;49:1458–1460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

