Single-molecule three-color FRET with both negligible spectral overlap and long observation time
- PMID: 20808851
- PMCID: PMC2924373
- DOI: 10.1371/journal.pone.0012270
Single-molecule three-color FRET with both negligible spectral overlap and long observation time
Abstract
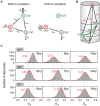
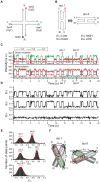
Full understanding of complex biological interactions frequently requires multi-color detection capability in doing single-molecule fluorescence resonance energy transfer (FRET) experiments. Existing single-molecule three-color FRET techniques, however, suffer from severe photobleaching of Alexa 488, or its alternative dyes, and have been limitedly used for kinetics studies. In this work, we developed a single-molecule three-color FRET technique based on the Cy3-Cy5-Cy7 dye trio, thus providing enhanced observation time and improved data quality. Because the absorption spectra of three fluorophores are well separated, real-time monitoring of three FRET efficiencies was possible by incorporating the alternating laser excitation (ALEX) technique both in confocal microscopy and in total-internal-reflection fluorescence (TIRF) microscopy.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

