Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei
- PMID: 20808868
- PMCID: PMC2924390
- DOI: 10.1371/journal.pone.0012294
Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei
Abstract
Background: The Plasmodium protein Cell-traversal protein for ookinetes and sporozoites (CelTOS) plays an important role in cell traversal of host cells in both, mosquito and vertebrates, and is required for successful malaria infections. CelTOS is highly conserved among the Plasmodium species, suggesting an important functional role across all species. Therefore, targeting the immune response to this highly conserved protein and thus potentially interfering with its biological function may result in protection against infection even by heterologous species of Plasmodium.
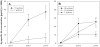
Methodology/principal findings: To test this hypothesis, we developed a recombinant codon-harmonized P. falciparum CelTOS protein that can be produced to high yields in the E. coli expression system. Inbred Balb/c and outbred CD-1 mice were immunized with various doses of the recombinant protein adjuvanted with Montanide ISA 720 and characterized using in vitro and in vivo analyses.
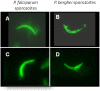
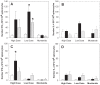
Conclusions/significance: Immunization with PfCelTOS resulted in potent humoral and cellular immune responses and most importantly induced sterile protection against a heterologous challenge with P. berghei sporozoites in a proportion of both inbred and outbred mice. The biological activity of CelTOS-specific antibodies against the malaria parasite is likely linked to the impairment of sporozoite motility and hepatocyte infectivity. The results underscore the potential of this antigen as a pre-erythrocytic vaccine candidate and demonstrate for the first time a malaria vaccine that is cross-protective between species.
Conflict of interest statement
Figures


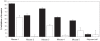
Similar articles
-
The Plasmodium falciparum Cell-Traversal Protein for Ookinetes and Sporozoites as a Candidate for Preerythrocytic and Transmission-Blocking Vaccines.Infect Immun. 2017 Jan 26;85(2):e00498-16. doi: 10.1128/IAI.00498-16. Print 2017 Feb. Infect Immun. 2017. PMID: 27895131 Free PMC article.
-
Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS.Vaccine. 2011 Aug 11;29(35):5940-9. doi: 10.1016/j.vaccine.2011.06.053. Epub 2011 Jun 29. Vaccine. 2011. PMID: 21722682
-
Measuring naturally acquired ex vivo IFN-γ responses to Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (CelTOS) in Ghanaian adults.Malar J. 2015 Jan 21;14:20. doi: 10.1186/s12936-014-0539-5. Malar J. 2015. PMID: 25604473 Free PMC article.
-
Exploring in vitro expression and immune potency in mice using mRNA encoding the Plasmodium falciparum malaria antigen, CelTOS.Front Immunol. 2022 Dec 15;13:1026052. doi: 10.3389/fimmu.2022.1026052. eCollection 2022. Front Immunol. 2022. PMID: 36591298 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
Immunological Cross-Reactivity between Malaria Vaccine Target Antigen P48/45 in Plasmodium vivax and P. falciparum and Cross-Boosting of Immune Responses.PLoS One. 2016 Jul 20;11(7):e0158212. doi: 10.1371/journal.pone.0158212. eCollection 2016. PLoS One. 2016. PMID: 27438603 Free PMC article.
-
Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria.Front Immunol. 2013 Jul 4;4:176. doi: 10.3389/fimmu.2013.00176. eCollection 2013. Front Immunol. 2013. PMID: 23847617 Free PMC article.
-
The case for a rational genome-based vaccine against malaria.Front Microbiol. 2015 Jan 22;5:741. doi: 10.3389/fmicb.2014.00741. eCollection 2014. Front Microbiol. 2015. PMID: 25657640 Free PMC article. Review.
-
Expression of Recombinant PfCelTOS Antigen in the Chloroplast of Chlamydomonas reinhardtii and its Potential Use in Detection of Malaria.Mol Biotechnol. 2019 Feb;61(2):102-110. doi: 10.1007/s12033-018-0140-1. Mol Biotechnol. 2019. PMID: 30506260
-
The Case for Exploiting Cross-Species Epitopes in Malaria Vaccine Design.Front Immunol. 2020 Feb 27;11:335. doi: 10.3389/fimmu.2020.00335. eCollection 2020. Front Immunol. 2020. PMID: 32174924 Free PMC article. Review.
References
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Efficacy of the RTS,S/ASO2A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. - PubMed
-
- Sacarlal J, Aide P, Aponte JJ, Renom M, Leach A, et al. Long-term safety and efficacy of the RTS,S/ASO2A malaria vaccine in Mozambican children. J Infect Dis. 2009;200:329–336. - PubMed
-
- Sharma S, Pahtak S. Malaria vaccine: a current perspective. J Vector Borne Dis. 2008;45:1–20. - PubMed
-
- Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by X-irradiated Plasmodium berghei sporozoites. Nature. 1969;222:488–489. - PubMed
-
- Sina BJ, Do Rosario VE, Woollett G, Sakhuja K, Hollingdale M. Plasmodium falciparum sporozoite immunization protections against Plasmodium berghei sporozoite infection. Experimental Parasitol. 1983;77:122–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

