Spike-timing theory of working memory
- PMID: 20808877
- PMCID: PMC2924241
- DOI: 10.1371/journal.pcbi.1000879
Spike-timing theory of working memory
Abstract
Working memory (WM) is the part of the brain's memory system that provides temporary storage and manipulation of information necessary for cognition. Although WM has limited capacity at any given time, it has vast memory content in the sense that it acts on the brain's nearly infinite repertoire of lifetime long-term memories. Using simulations, we show that large memory content and WM functionality emerge spontaneously if we take the spike-timing nature of neuronal processing into account. Here, memories are represented by extensively overlapping groups of neurons that exhibit stereotypical time-locked spatiotemporal spike-timing patterns, called polychronous patterns; and synapses forming such polychronous neuronal groups (PNGs) are subject to associative synaptic plasticity in the form of both long-term and short-term spike-timing dependent plasticity. While long-term potentiation is essential in PNG formation, we show how short-term plasticity can temporarily strengthen the synapses of selected PNGs and lead to an increase in the spontaneous reactivation rate of these PNGs. This increased reactivation rate, consistent with in vivo recordings during WM tasks, results in high interspike interval variability and irregular, yet systematically changing, elevated firing rate profiles within the neurons of the selected PNGs. Additionally, our theory explains the relationship between such slowly changing firing rates and precisely timed spikes, and it reveals a novel relationship between WM and the perception of time on the order of seconds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
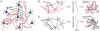
 different groups.
different groups.
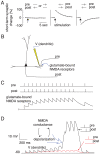
 seconds (see Methods). (A) Top: Average multiunit firing rate and short-term synaptic change for tPNG (red) and for the rest of the excitatory neurons (blue). The green curve illustrates how the short-term change would decay back to baseline in the absence of neural activity after stimulation. (B) Magnified spike rasters of two partial reactivations of the tPNG neurons at two different times: Red dots, spikes of tPNG neurons; Circles, expected firings (see Methods) of all neurons in the tPNG. Only neurons belonging to the tPNG are shown. (C) CV, inter-spike interval variability histogram for tPNG neurons: Red, tPNG in WM (notice high CV values); Blue, spontaneous network activity, no PNG in WM (spike raster not shown). (D) Cross-correlograms of two neurons from the tPNG: Red, tPNG in WM; Blue, spontaneous network activity. (E) Average firing rate histogram of three representative tPNG neurons (red) while the tPNG in WM, and of a control neuron (blue) from the rest of the network. (F) Histogram of the duration of PNGs put separately in WM: time of the last reactivation (after the offset of stimulation) of each PNG versus number of PNGs with a given maximum reactivation span.
seconds (see Methods). (A) Top: Average multiunit firing rate and short-term synaptic change for tPNG (red) and for the rest of the excitatory neurons (blue). The green curve illustrates how the short-term change would decay back to baseline in the absence of neural activity after stimulation. (B) Magnified spike rasters of two partial reactivations of the tPNG neurons at two different times: Red dots, spikes of tPNG neurons; Circles, expected firings (see Methods) of all neurons in the tPNG. Only neurons belonging to the tPNG are shown. (C) CV, inter-spike interval variability histogram for tPNG neurons: Red, tPNG in WM (notice high CV values); Blue, spontaneous network activity, no PNG in WM (spike raster not shown). (D) Cross-correlograms of two neurons from the tPNG: Red, tPNG in WM; Blue, spontaneous network activity. (E) Average firing rate histogram of three representative tPNG neurons (red) while the tPNG in WM, and of a control neuron (blue) from the rest of the network. (F) Histogram of the duration of PNGs put separately in WM: time of the last reactivation (after the offset of stimulation) of each PNG versus number of PNGs with a given maximum reactivation span.
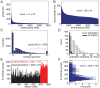
 sec). Black, middle: cross-correlograms of neurons that are part of the black but not the red PNG, when only the black PNG is in WM (spike raster not shown). Right: cross-correlograms of two neurons, one from each target PNG, when both PNGs are in WM (
sec). Black, middle: cross-correlograms of neurons that are part of the black but not the red PNG, when only the black PNG is in WM (spike raster not shown). Right: cross-correlograms of two neurons, one from each target PNG, when both PNGs are in WM ( sec).
sec).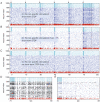
 . (C) Short-term STDP blocked when
. (C) Short-term STDP blocked when  . (A,B,C) Identical conditions when
. (A,B,C) Identical conditions when  . (D, E) The [74 … 83] second segment of the spike raster data of A and B are magnified in D and E, respectively. (A,D) In the presence of sufficient non-specific drive and short-term STDP, after repeated presentations a new PNG — representing the novel cue — emerges and gets frequently activated (about 4 Hz). (D) Neurons that became part of the new PNG initiated by the spiking of red neurons are marked black. The new group consists of 24 (out of 60) red and 118 black excitatory neurons. Notice that 36 of the stimulated red neurons did not become part of the newly formed PNG probably due to the lack of appropriate synaptic connections. (B,E,C) Hardly any replay in B and E, and no replay at all in C. Hampered PNG formation as WM mechanism was prevented.
. (D, E) The [74 … 83] second segment of the spike raster data of A and B are magnified in D and E, respectively. (A,D) In the presence of sufficient non-specific drive and short-term STDP, after repeated presentations a new PNG — representing the novel cue — emerges and gets frequently activated (about 4 Hz). (D) Neurons that became part of the new PNG initiated by the spiking of red neurons are marked black. The new group consists of 24 (out of 60) red and 118 black excitatory neurons. Notice that 36 of the stimulated red neurons did not become part of the newly formed PNG probably due to the lack of appropriate synaptic connections. (B,E,C) Hardly any replay in B and E, and no replay at all in C. Hampered PNG formation as WM mechanism was prevented.References
-
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–4. - PubMed
-
- Baddeley AD, Hitch G. Bower GA, editor. Working memory, New York: Academic Press, volume 8 of. The psychology of learning and motivation: advances in research and theory. 1974. pp. 47–90.
-
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–49. - PubMed
-
- Baddeley A. Working memory. Scholarpedia J. 2010;5(2):3015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

