A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island
- PMID: 20808891
- PMCID: PMC2924317
- DOI: 10.1371/journal.pgen.1001069
A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island
Abstract
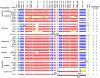
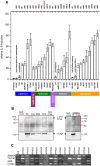
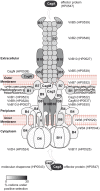
The Helicobacter pylori cag pathogenicity island (cagPAI) encodes a type IV secretion system. Humans infected with cagPAI-carrying H. pylori are at increased risk for sequelae such as gastric cancer. Housekeeping genes in H. pylori show considerable genetic diversity; but the diversity of virulence factors such as the cagPAI, which transports the bacterial oncogene CagA into host cells, has not been systematically investigated. Here we compared the complete cagPAI sequences for 38 representative isolates from all known H. pylori biogeographic populations. Their gene content and gene order were highly conserved. The phylogeny of most cagPAI genes was similar to that of housekeeping genes, indicating that the cagPAI was probably acquired only once by H. pylori, and its genetic diversity reflects the isolation by distance that has shaped this bacterial species since modern humans migrated out of Africa. Most isolates induced IL-8 release in gastric epithelial cells, indicating that the function of the Cag secretion system has been conserved despite some genetic rearrangements. More than one third of cagPAI genes, in particular those encoding cell-surface exposed proteins, showed signatures of diversifying (Darwinian) selection at more than 5% of codons. Several unknown gene products predicted to be under Darwinian selection are also likely to be secreted proteins (e.g. HP0522, HP0535). One of these, HP0535, is predicted to code for either a new secreted candidate effector protein or a protein which interacts with CagA because it contains two genetic lineages, similar to cagA. Our study provides a resource that can guide future research on the biological roles and host interactions of cagPAI proteins, including several whose function is still unknown.
Conflict of interest statement
MV is an employee of Applied Maths nv and therefore has competing interest for the Kodon software.
Figures




Similar articles
-
Helicobacter pylori with East Asian-type cagPAI genes is more virulent than strains with Western-type in some cagPAI genes.Braz J Microbiol. 2017 Apr-Jun;48(2):218-224. doi: 10.1016/j.bjm.2016.12.004. Epub 2016 Dec 22. Braz J Microbiol. 2017. PMID: 28130020 Free PMC article.
-
Helicobacter pylori cag pathogenicity island genotype diversity within the gastric niche of a single host.J Med Microbiol. 2007 May;56(Pt 5):664-669. doi: 10.1099/jmm.0.46885-0. J Med Microbiol. 2007. PMID: 17446291
-
Evaluation of the effect of cagPAI genes of Helicobacter pylori on AGS epithelial cell morphology and IL-8 secretion.Antonie Van Leeuwenhoek. 2014 Jan;105(1):179-89. doi: 10.1007/s10482-013-0064-5. Epub 2013 Oct 30. Antonie Van Leeuwenhoek. 2014. PMID: 24170115
-
Roles of the components of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori.Future Microbiol. 2024;19(14):1253-1267. doi: 10.1080/17460913.2024.2383514. Epub 2024 Aug 22. Future Microbiol. 2024. PMID: 39171625 Free PMC article. Review.
-
The type IV secretion system in Helicobacter pylori.Future Microbiol. 2018 Jul;13:1041-1054. doi: 10.2217/fmb-2018-0038. Epub 2018 Jun 21. Future Microbiol. 2018. PMID: 29927340 Review.
Cited by
-
Immunobiological effects of lipopolysaccharide derived from Helicobacter pylori and influence of a proton pump inhibitor lansoprazole on human polymorphonuclear leukocytes.Folia Microbiol (Praha). 2024 Dec;69(6):1369-1378. doi: 10.1007/s12223-024-01188-7. Epub 2024 Aug 17. Folia Microbiol (Praha). 2024. PMID: 39153156 Free PMC article.
-
Bacterial Energetic Requirements for Helicobacter pylori Cag Type IV Secretion System-Dependent Alterations in Gastric Epithelial Cells.Infect Immun. 2020 Jan 22;88(2):e00790-19. doi: 10.1128/IAI.00790-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31712269 Free PMC article.
-
Draft Genome Sequences of Helicobacter pylori Strains HPARG63 and HPARG8G, Cultured from Patients with Chronic Gastritis and Gastric Ulcer Disease.Genome Announc. 2013 Sep 5;1(5):e00700-13. doi: 10.1128/genomeA.00700-13. Genome Announc. 2013. PMID: 24009126 Free PMC article.
-
Characterization of the Cag pathogenicity island in Helicobacter pylori from naturally infected rhesus macaques.FEMS Microbiol Lett. 2016 Dec;363(24):fnw275. doi: 10.1093/femsle/fnw275. Epub 2016 Dec 8. FEMS Microbiol Lett. 2016. PMID: 27940463 Free PMC article.
-
Helicobacter pylori Modulates Heptose Metabolite Biosynthesis and Heptose-Dependent Innate Immune Host Cell Activation by Multiple Mechanisms.Microbiol Spectr. 2023 Jun 15;11(3):e0313222. doi: 10.1128/spectrum.03132-22. Epub 2023 Apr 27. Microbiol Spectr. 2023. PMID: 37129481 Free PMC article.
References
-
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. - PubMed
-
- Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreit S, et al. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–1348. - PubMed
-
- Wiedemann T, Loell E, Mueller S, Stoeckelhuber M, Stolte M, et al. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS ONE. 2009;4:e4754. doi: 10.1371/journal.pone.0004754. - DOI - PMC - PubMed
-
- Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

