The synthetic bacterial lipopeptide Pam3CSK4 modulates respiratory syncytial virus infection independent of TLR activation
- PMID: 20808895
- PMCID: PMC2924323
- DOI: 10.1371/journal.ppat.1001049
The synthetic bacterial lipopeptide Pam3CSK4 modulates respiratory syncytial virus infection independent of TLR activation
Abstract
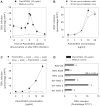
Respiratory syncytial virus (RSV) is an important cause of acute respiratory disease in infants, immunocompromised subjects and the elderly. However, it is unclear why most primary RSV infections are associated with relatively mild symptoms, whereas some result in severe lower respiratory tract infections and bronchiolitis. Since RSV hospitalization has been associated with respiratory bacterial co-infections, we have tested if bacterial Toll-like receptor (TLR) agonists influence RSV-A2-GFP infection in human primary cells or cell lines. The synthetic bacterial lipopeptide Pam3-Cys-Ser-Lys4 (Pam3CSK4), the prototype ligand for the heterodimeric TLR1/TLR2 complex, enhanced RSV infection in primary epithelial, myeloid and lymphoid cells. Surprisingly, enhancement was optimal when lipopeptides and virus were added simultaneously, whereas addition of Pam3CSK4 immediately after infection had no effect. We have identified two structurally related lipopeptides without TLR-signaling capacity that also modulate RSV infection, whereas Pam3CSK4-reminiscent TLR1/2 agonists did not, and conclude that modulation of infection is independent of TLR activation. A similar TLR-independent enhancement of infection could also be demonstrated for wild-type RSV strains, and for HIV-1, measles virus and human metapneumovirus. We show that the effect of Pam3CSK4 is primarily mediated by enhanced binding of RSV to its target cells. The N-palmitoylated cysteine and the cationic lysines were identified as pivotal for enhanced virus binding. Surprisingly, we observed inhibition of RSV infection in immortalized epithelial cell lines, which was shown to be related to interactions between Pam3CSK4 and negatively charged glycosaminoglycans on these cells, which are known targets for binding of laboratory-adapted but not wild-type RSV. These data suggest a potential role for bacterial lipopeptides in enhanced binding of RSV and other viruses to their target cells, thus affecting viral entry or spread independent of TLR signaling. Moreover, our results also suggest a potential application for these synthetic lipopeptides as adjuvants for live-attenuated viral vaccines.
Conflict of interest statement
Prof. K.-H. Wiesmüller is CEO of the company EMC microcollections GmbH. Besides this he is professor at the University of Tübingen. His affiliation with EMC does not alter the adherence to all PLoS policies on sharing data and materials. Any materials and information associated with our publication can be made freely available upon reasonable request for the purpose of academic, non-commercial research. A.D.M.E. Osterhaus founded and is chief scientific officer of ViroClinics, a company set up in collaboration with Erasmus University. However, for clarification, no materials or support were received from the company, and no agreements were in place concerning the execution or publication of this work. All authors declare they don't have any financial, personal, or professional interests that compromise the work, its interpretation or influence.
Figures


Similar articles
-
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies.J Virol. 2020 Sep 29;94(20):e01068-20. doi: 10.1128/JVI.01068-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759319 Free PMC article.
-
CpG in Combination with an Inhibitor of Notch Signaling Suppresses Formalin-Inactivated Respiratory Syncytial Virus-Enhanced Airway Hyperresponsiveness and Inflammation by Inhibiting Th17 Memory Responses and Promoting Tissue-Resident Memory Cells in Lungs.J Virol. 2017 Apr 28;91(10):e02111-16. doi: 10.1128/JVI.02111-16. Print 2017 May 15. J Virol. 2017. PMID: 28275186 Free PMC article.
-
Lipopolysaccharide Inhibits FI-RSV Vaccine-enhanced Inflammation Through Regulating Th Responses.Curr Med Sci. 2019 Jun;39(3):363-370. doi: 10.1007/s11596-019-2044-0. Epub 2019 Jun 17. Curr Med Sci. 2019. PMID: 31209804
-
Respiratory syncytial virus infection and the tight junctions of nasal epithelial cells.Adv Otorhinolaryngol. 2011;72:153-6. doi: 10.1159/000324777. Epub 2011 Aug 18. Adv Otorhinolaryngol. 2011. PMID: 21865717 Review.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
Cited by
-
Infection-enhancing lipopeptides do not improve intranasal immunization of cotton rats with a delta-G candidate live-attenuated human respiratory syncytial virus vaccine.Hum Vaccin Immunother. 2013 Dec;9(12):2578-83. doi: 10.4161/hv.26096. Epub 2013 Aug 16. Hum Vaccin Immunother. 2013. PMID: 23955280 Free PMC article.
-
Lipopeptide PAM3CYS4 Synergizes N-Formyl-Met-Leu-Phe (fMLP)-Induced Calcium Transients in Mouse Neutrophils.Shock. 2018 Oct;50(4):493-499. doi: 10.1097/SHK.0000000000001062. Shock. 2018. PMID: 29176405 Free PMC article.
-
Effects of over-expression of TLR2 in transgenic goats on pathogen clearance and role of up-regulation of lysozyme secretion and infiltration of inflammatory cells.BMC Vet Res. 2012 Oct 22;8:196. doi: 10.1186/1746-6148-8-196. BMC Vet Res. 2012. PMID: 23082910 Free PMC article.
-
Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells.J Immunol. 2014 May 1;192(9):4233-41. doi: 10.4049/jimmunol.1302850. Epub 2014 Mar 28. J Immunol. 2014. PMID: 24683188 Free PMC article.
-
Delineating morbillivirus entry, dissemination and airborne transmission by studying in vivo competition of multicolor canine distemper viruses in ferrets.PLoS Pathog. 2017 May 8;13(5):e1006371. doi: 10.1371/journal.ppat.1006371. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28481926 Free PMC article.
References
-
- Collins PL, Crowe JE., Jr . Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1601–1646.
-
- Chanock RM, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957;66:281–290. - PubMed
-
- Chanock RM, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg. 1957;66:291–300. - PubMed
-
- Neilson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. - PubMed
-
- Simoes EAF. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

