Disease-associated mutations that alter the RNA structural ensemble
- PMID: 20808897
- PMCID: PMC2924325
- DOI: 10.1371/journal.pgen.1001074
Disease-associated mutations that alter the RNA structural ensemble
Abstract
Genome-wide association studies (GWAS) often identify disease-associated mutations in intergenic and non-coding regions of the genome. Given the high percentage of the human genome that is transcribed, we postulate that for some observed associations the disease phenotype is caused by a structural rearrangement in a regulatory region of the RNA transcript. To identify such mutations, we have performed a genome-wide analysis of all known disease-associated Single Nucleotide Polymorphisms (SNPs) from the Human Gene Mutation Database (HGMD) that map to the untranslated regions (UTRs) of a gene. Rather than using minimum free energy approaches (e.g. mFold), we use a partition function calculation that takes into consideration the ensemble of possible RNA conformations for a given sequence. We identified in the human genome disease-associated SNPs that significantly alter the global conformation of the UTR to which they map. For six disease-states (Hyperferritinemia Cataract Syndrome, beta-Thalassemia, Cartilage-Hair Hypoplasia, Retinoblastoma, Chronic Obstructive Pulmonary Disease (COPD), and Hypertension), we identified multiple SNPs in UTRs that alter the mRNA structural ensemble of the associated genes. Using a Boltzmann sampling procedure for sub-optimal RNA structures, we are able to characterize and visualize the nature of the conformational changes induced by the disease-associated mutations in the structural ensemble. We observe in several cases (specifically the 5' UTRs of FTL and RB1) SNP-induced conformational changes analogous to those observed in bacterial regulatory Riboswitches when specific ligands bind. We propose that the UTR and SNP combinations we identify constitute a "RiboSNitch," that is a regulatory RNA in which a specific SNP has a structural consequence that results in a disease phenotype. Our SNPfold algorithm can help identify RiboSNitches by leveraging GWAS data and an analysis of the mRNA structural ensemble.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
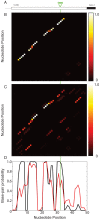

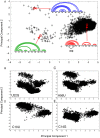
Comment in
-
Disease genetics: SNPs and the structural deficit.Nat Rev Genet. 2010 Oct;11(10):669. doi: 10.1038/nrg2871. Epub 2010 Sep 7. Nat Rev Genet. 2010. PMID: 20820181 No abstract available.
Similar articles
-
Structural effects of linkage disequilibrium on the transcriptome.RNA. 2012 Jan;18(1):77-87. doi: 10.1261/rna.029900.111. Epub 2011 Nov 22. RNA. 2012. PMID: 22109839 Free PMC article.
-
The potential of the riboSNitch in personalized medicine.Wiley Interdiscip Rev RNA. 2015 Sep-Oct;6(5):517-32. doi: 10.1002/wrna.1291. Epub 2015 Jun 26. Wiley Interdiscip Rev RNA. 2015. PMID: 26115028 Free PMC article. Review.
-
In silico whole-genome screening for cancer-related single-nucleotide polymorphisms located in human mRNA untranslated regions.BMC Genomics. 2007 Jan 3;8:2. doi: 10.1186/1471-2164-8-2. BMC Genomics. 2007. PMID: 17201911 Free PMC article.
-
Comparative Visualization of the RNA Suboptimal Conformational Ensemble In Vivo.Biophys J. 2017 Jul 25;113(2):290-301. doi: 10.1016/j.bpj.2017.05.031. Epub 2017 Jun 15. Biophys J. 2017. PMID: 28625696 Free PMC article.
-
Variation in the Untranslated Genome and Susceptibility to Infections.Front Immunol. 2018 Sep 7;9:2046. doi: 10.3389/fimmu.2018.02046. eCollection 2018. Front Immunol. 2018. PMID: 30245696 Free PMC article. Review.
Cited by
-
Efficient procedures for the numerical simulation of mid-size RNA kinetics.Algorithms Mol Biol. 2012 Sep 7;7(1):24. doi: 10.1186/1748-7188-7-24. Algorithms Mol Biol. 2012. PMID: 22958879 Free PMC article.
-
Analysis of transcript-deleterious variants in Mendelian disorders: implications for RNA-based diagnostics.Genome Biol. 2020 Jun 17;21(1):145. doi: 10.1186/s13059-020-02053-9. Genome Biol. 2020. PMID: 32552793 Free PMC article. Clinical Trial.
-
Evaluating our ability to predict the structural disruption of RNA by SNPs.BMC Genomics. 2012 Jun 18;13 Suppl 4(Suppl 4):S6. doi: 10.1186/1471-2164-13-S4-S6. BMC Genomics. 2012. PMID: 22759654 Free PMC article.
-
How does precursor RNA structure influence RNA processing and gene expression?Biosci Rep. 2023 Mar 31;43(3):BSR20220149. doi: 10.1042/BSR20220149. Biosci Rep. 2023. PMID: 36689327 Free PMC article. Review.
-
RNA Secondary Structure Alteration Caused by Single Nucleotide Variants.Methods Mol Biol. 2023;2586:107-120. doi: 10.1007/978-1-0716-2768-6_7. Methods Mol Biol. 2023. PMID: 36705901
References
-
- Morton NE. Into the post-HapMap era. Adv Genet. 2008;60:727–742. - PubMed
-
- Mathew CG. New links to the pathogenesis of Crohn disease provided by genome-wide association scans. Nat Rev Genet. 2008;9:9–14. - PubMed
-
- Lee SH, van der Werf JH, Hayes BJ, Goddard ME, Visscher PM. Predicting unobserved phenotypes for complex traits from whole-genome SNP data. PLoS Genet. 2008;4:e1000231. doi: 10.1371/journal.pgen.1000231. - DOI - PMC - PubMed
-
- Lee ST, Choi KW, Yeo HT, Kim JW, Ki CS, et al. Identification of an Arg35X mutation in the PDCD10 gene in a patient with cerebral and multiple spinal cavernous malformations. J Neurol Sci. 2008;267:177–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

