The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset
- PMID: 20809212
- PMCID: PMC3045510
- DOI: 10.1007/s00213-010-1989-2
The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset
Abstract
Rationale: Exposure of the immature mammalian brain to stress factors, including stress levels of glucocorticoids, either prenatally or postnatally, is regarded as a major regulatory factor in short- and long-term brain function and, in human, as a major aetiological factor in neuropsychiatric disorders. Experimental human studies are not feasible and animal studies are required to demonstrate causality and elucidate mechanisms. A number of studies have been conducted and reviewed in rodents but there are relatively few studies in primates.
Objectives: Here we present an overview of our published studies and some original data on the effects of: (1) prenatal stress on hypothalamic-pituitary-adrenal (HPA) re/activity and hippocampus neuroanatomy in juvenile-adolescent rhesus macaques; (2) prenatal dexamethasone (DEX) on HPA activity, behaviour and prefrontal cortex neuroanatomy in infant-adolescent common marmosets; (3) postnatal daily parental separation stress on HPA re/activity, behaviour, sleep and hippocampus and prefrontal cortex neuroanatomy in infant-adolescent common marmoset.
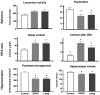
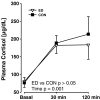
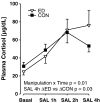
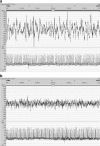
Results: Prenatal stress increased basal cortisol levels and reduced neurogenesis in macaque. Prenatal DEX was without effect on HPA activity and reduced social play and skilled motor behaviour in marmoset. Postnatal social stress increased basal cortisol levels, reduced social play, increased awakening and reduced hippocampal glucocorticoid and mineralocorticoid receptor expression in marmoset.
Conclusions: Perinatal stress-related environmental events exert short- and long-term effects on HPA function, behaviour and brain status in rhesus macaque and common marmoset. The mechanisms mediating the enduring effects remain to be elucidated, with candidates including increased basal HPA function and epigenetic programming.
Figures
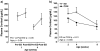
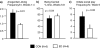

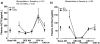
Similar articles
-
Prenatal dexamethasone prevents early and long-lasting neuroendocrine and behavioral effects of maternal stress on male offspring.Fiziol Zh (1994). 2008;54(5):28-39. Fiziol Zh (1994). 2008. PMID: 19058510
-
Gestational cortisol and social play shape development of marmosets' HPA functioning and behavioral responses to stressors.Dev Psychobiol. 2014 Sep;56(6):1229-43. doi: 10.1002/dev.21203. Epub 2014 Feb 10. Dev Psychobiol. 2014. PMID: 24510474 Free PMC article.
-
Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys.Endocrinology. 2008 Dec;149(12):6343-55. doi: 10.1210/en.2008-0615. Epub 2008 Aug 28. Endocrinology. 2008. PMID: 18755792
-
Neurotoxicity of glucocorticoids in the primate brain.Horm Behav. 1994 Dec;28(4):336-48. doi: 10.1006/hbeh.1994.1030. Horm Behav. 1994. PMID: 7729802 Review.
-
Prenatal stress alters early neurobehavior, stress reactivity and learning in non-human primates: a brief review.Stress. 2001 Sep;4(3):183-93. doi: 10.3109/10253890109035017. Stress. 2001. PMID: 22432139 Review.
Cited by
-
Does growth impairment underlie the adverse effects of dexamethasone on development of noradrenergic systems?Toxicology. 2018 Sep 1;408:11-21. doi: 10.1016/j.tox.2018.06.008. Epub 2018 Jun 20. Toxicology. 2018. PMID: 29935188 Free PMC article.
-
Pregestational Prediabetes Induces Maternal Hypothalamic-Pituitary-Adrenal (HPA) Axis Dysregulation and Results in Adverse Foetal Outcomes.Int J Mol Sci. 2024 May 16;25(10):5431. doi: 10.3390/ijms25105431. Int J Mol Sci. 2024. PMID: 38791468 Free PMC article.
-
Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis.Neuropsychopharmacology. 2013 Apr;38(5):872-83. doi: 10.1038/npp.2012.253. Epub 2012 Dec 6. Neuropsychopharmacology. 2013. PMID: 23303060 Free PMC article.
-
Adult-like action potential properties and abundant GABAergic synaptic responses in amygdala neurons from newborn marmosets.J Physiol. 2012 Nov 15;590(22):5691-706. doi: 10.1113/jphysiol.2012.235010. Epub 2012 Sep 10. J Physiol. 2012. PMID: 22966158 Free PMC article.
-
Prenatal and postnatal inflammation in relation to cortisol levels in preterm infants at 18 months corrected age.J Perinatol. 2013 Aug;33(8):647-51. doi: 10.1038/jp.2013.24. Epub 2013 Apr 4. J Perinatol. 2013. PMID: 23558431 Free PMC article.
References
-
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, Pryce CR. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol Psychiatry. 2010;67(11):1106–1109. - PubMed
-
- Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–246. - PubMed
-
- Aubert Y. Effects of early life stress on ECoG-defined sleep patterns in the common marmoset monkey biology department. Zürich: Swiss Federal Institute of Technology Zürich; 2004.
-
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders—a meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

