Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth
- PMID: 20809552
- PMCID: PMC3043358
- DOI: 10.1002/pros.21247
Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth
Abstract
Background: The clinical success of the nucleoside analogs 5-aza-cytidine (5-azaC) and 5-aza-2'deoxycytidine (5-aza-dC) as DNA methyltransferase (DNMT) inhibitors has spurred interest in the development of non-nucleoside inhibitors with improved pharmacologic and safety profiles. Because DNMT catalysis features attack of cytosine bases by an enzyme thiol group, we tested whether disulfiram (DSF), a thiol-reactive compound with known clinical safety, demonstrated DNMT inhibitory activity.
Methods: Inhibition of DNMT1 activity by DSF was assessed using methyltransferase activity assays with recombinant DNMT1. Next, prostate cancer cell lines were exposed to DSF and assessed for: i) reduction of global 5-methyl cytosine ((5me)C) content using liquid chromatography/tandem mass spectrometry (LC-MS/MS); ii) gene-specific promoter demethylation by methylation-specific PCR (MSP); and iii) gene-reactivation by real-time RT-PCR. DSF was also tested for growth inhibition using prostate cancer cell lines propagated in vitro in cell culture and in vivo as xenografts in nude mice.
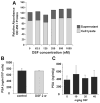
Results: Disulfiram showed a dose-dependent inhibition of DNMT1 activity on a hemimethylated DNA substrate. In prostate cancer cells in culture, DSF exposure led to reduction of global genomic (5me)C content, increase in unmethylated APC and RARB gene promoters, and associated re-expression of these genes, but did not significantly alter prostate-specific antigen (PSA) expression. DSF significantly inhibited growth and clonogenic survival of prostate cancer cell lines in culture and showed a trend for reduced growth of prostate cancer xenografts.
Conclusions: Disulfiram is a non-nucleoside DNMT1 inhibitor that can reduce global (5me)C content, reactivate epigenetically silenced genes, and significantly inhibit growth in prostate cancer cell lines.
Copyright © 2010 Wiley-Liss, Inc.
Figures

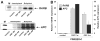

Similar articles
-
Disulfiram and its novel derivative sensitize prostate cancer cells to the growth regulatory mechanisms of the cell by re-expressing the epigenetically repressed tumor suppressor-estrogen receptor β.Mol Carcinog. 2016 Nov;55(11):1843-1857. doi: 10.1002/mc.22433. Epub 2015 Nov 24. Mol Carcinog. 2016. PMID: 26599461
-
Anti-tumoral effect of the non-nucleoside DNMT inhibitor RG108 in human prostate cancer cells.Curr Pharm Des. 2014;20(11):1803-11. doi: 10.2174/13816128113199990516. Curr Pharm Des. 2014. PMID: 23888969
-
Activation of silenced tumor suppressor genes in prostate cancer cells by a novel energy restriction-mimetic agent.Prostate. 2012 Dec 1;72(16):1767-78. doi: 10.1002/pros.22530. Epub 2012 Apr 26. Prostate. 2012. PMID: 22539223 Free PMC article.
-
Antineoplastic activity of the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine in anaplastic large cell lymphoma.Biochimie. 2012 Nov;94(11):2297-307. doi: 10.1016/j.biochi.2012.05.029. Epub 2012 Jun 9. Biochimie. 2012. PMID: 22687603 Free PMC article. Review.
-
DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer.Curr Cancer Drug Targets. 2013 May;13(4):379-99. doi: 10.2174/15680096113139990077. Curr Cancer Drug Targets. 2013. PMID: 23517596 Review.
Cited by
-
Inhibitors of DNA Methylation.Adv Exp Med Biol. 2022;1389:471-513. doi: 10.1007/978-3-031-11454-0_17. Adv Exp Med Biol. 2022. PMID: 36350520 Review.
-
Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells.Mini Rev Med Chem. 2012 Oct;12(12):1193-201. doi: 10.2174/138955712802762040. Mini Rev Med Chem. 2012. PMID: 22931591 Free PMC article.
-
Targeting DNA Methyltranferases in Urological Tumors.Front Pharmacol. 2018 Apr 13;9:366. doi: 10.3389/fphar.2018.00366. eCollection 2018. Front Pharmacol. 2018. PMID: 29706891 Free PMC article. Review.
-
Silencing DNA methyltransferase 1 leads to the activation of the esophageal suppressor gene p16 in vitro and in vivo.Oncol Lett. 2017 Sep;14(3):3077-3081. doi: 10.3892/ol.2017.6535. Epub 2017 Jul 7. Oncol Lett. 2017. PMID: 28927055 Free PMC article.
-
Targeting Aldehyde Dehydrogenases to Eliminate Cancer Stem Cells in Gynecologic Malignancies.Cancers (Basel). 2020 Apr 13;12(4):961. doi: 10.3390/cancers12040961. Cancers (Basel). 2020. PMID: 32295073 Free PMC article. Review.
References
-
- Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64(6):1975–1986. - PubMed
-
- Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese TL, Isaacs WB, Bova GS, De Marzo AM, Nelson WG. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68(21):8954–8967. - PMC - PubMed
-
- Nelson WG, Yegnasubramanian S, Agoston AT, Bastian PJ, Lee BH, Nakayama M, De Marzo AM. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–4266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

