Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth
- PMID: 20809552
- PMCID: PMC3043358
- DOI: 10.1002/pros.21247
Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth
Abstract
Background: The clinical success of the nucleoside analogs 5-aza-cytidine (5-azaC) and 5-aza-2'deoxycytidine (5-aza-dC) as DNA methyltransferase (DNMT) inhibitors has spurred interest in the development of non-nucleoside inhibitors with improved pharmacologic and safety profiles. Because DNMT catalysis features attack of cytosine bases by an enzyme thiol group, we tested whether disulfiram (DSF), a thiol-reactive compound with known clinical safety, demonstrated DNMT inhibitory activity.
Methods: Inhibition of DNMT1 activity by DSF was assessed using methyltransferase activity assays with recombinant DNMT1. Next, prostate cancer cell lines were exposed to DSF and assessed for: i) reduction of global 5-methyl cytosine ((5me)C) content using liquid chromatography/tandem mass spectrometry (LC-MS/MS); ii) gene-specific promoter demethylation by methylation-specific PCR (MSP); and iii) gene-reactivation by real-time RT-PCR. DSF was also tested for growth inhibition using prostate cancer cell lines propagated in vitro in cell culture and in vivo as xenografts in nude mice.
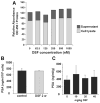
Results: Disulfiram showed a dose-dependent inhibition of DNMT1 activity on a hemimethylated DNA substrate. In prostate cancer cells in culture, DSF exposure led to reduction of global genomic (5me)C content, increase in unmethylated APC and RARB gene promoters, and associated re-expression of these genes, but did not significantly alter prostate-specific antigen (PSA) expression. DSF significantly inhibited growth and clonogenic survival of prostate cancer cell lines in culture and showed a trend for reduced growth of prostate cancer xenografts.
Conclusions: Disulfiram is a non-nucleoside DNMT1 inhibitor that can reduce global (5me)C content, reactivate epigenetically silenced genes, and significantly inhibit growth in prostate cancer cell lines.
Copyright © 2010 Wiley-Liss, Inc.
Figures

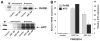

References
-
- Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64(6):1975–1986. - PubMed
-
- Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese TL, Isaacs WB, Bova GS, De Marzo AM, Nelson WG. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68(21):8954–8967. - PMC - PubMed
-
- Nelson WG, Yegnasubramanian S, Agoston AT, Bastian PJ, Lee BH, Nakayama M, De Marzo AM. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–4266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

