Efficient bioconjugation of protein capture agents to biosensor surfaces using aniline-catalyzed hydrazone ligation
- PMID: 20809595
- PMCID: PMC2947609
- DOI: 10.1021/la1021824
Efficient bioconjugation of protein capture agents to biosensor surfaces using aniline-catalyzed hydrazone ligation
Abstract
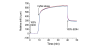
Aniline-catalyzed hydrazone ligation between surface-immobilized hydrazines and aldehyde-modified antibodies is shown to be an efficient method for attaching protein capture agents to model oxide-coated biosensor substrates. Silicon photonic microring resonators are used to directly evaluate the efficiency of this surface bioconjugate reaction at various pHs and in the presence or absence of aniline as a nucleophilic catalyst. It is found that aniline significantly increases the net antibody loading for surfaces functionalized over a pH range from 4.5 to 7.4, allowing derivatization of substrates with reduced incubation time and sample consumption. This increase in antibody loading directly results in more sensitive antigen detection when functionalized microrings are employed in a label-free immunoassay. Furthermore, these experiments also reveal an interesting pH-dependent noncovalent binding trend that plays an important role in dictating the amount of antibody attached onto the substrate, highlighting the competing contributions of the bioconjugate reaction rate and the dynamic interactions that control opportunities for a solution-phase biomolecule to react with a substrate-bound reagent.
Figures
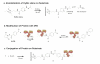
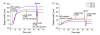
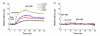
Similar articles
-
A highly efficient catalyst for oxime ligation and hydrazone-oxime exchange suitable for bioconjugation.Bioconjug Chem. 2013 Mar 20;24(3):333-42. doi: 10.1021/bc3004167. Epub 2013 Mar 6. Bioconjug Chem. 2013. PMID: 23425124 Free PMC article.
-
Nucleophilic catalysis of hydrazone formation and transimination: implications for dynamic covalent chemistry.J Am Chem Soc. 2006 Dec 13;128(49):15602-3. doi: 10.1021/ja067189k. J Am Chem Soc. 2006. PMID: 17147365
-
Insights into the mechanism and catalysis of oxime coupling chemistry at physiological pH.Chemistry. 2015 Apr 7;21(15):5980-5. doi: 10.1002/chem.201406458. Epub 2015 Mar 3. Chemistry. 2015. PMID: 25737419
-
Aniline-terminated DNA catalyzes rapid DNA-hydrazone formation at physiological pH.Chem Commun (Camb). 2014 Apr 14;50(29):3831-3. doi: 10.1039/c4cc00292j. Epub 2014 Mar 4. Chem Commun (Camb). 2014. PMID: 24590233
-
Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis.Chem Rev. 2017 Aug 9;117(15):10358-10376. doi: 10.1021/acs.chemrev.7b00090. Epub 2017 Jun 22. Chem Rev. 2017. PMID: 28640998 Free PMC article. Review.
Cited by
-
Aptamers as a sensitive tool to detect subtle modifications in therapeutic proteins.PLoS One. 2012;7(2):e31948. doi: 10.1371/journal.pone.0031948. Epub 2012 Feb 27. PLoS One. 2012. PMID: 22384109 Free PMC article.
-
Peptide-functionalized oxime hydrogels with tunable mechanical properties and gelation behavior.Biomacromolecules. 2013 Oct 14;14(10):3749-58. doi: 10.1021/bm401133r. Epub 2013 Oct 3. Biomacromolecules. 2013. PMID: 24050500 Free PMC article.
-
Development of an Interaction Assay between Single-Stranded Nucleic Acids Trapped with Silica Particles and Fluorescent Compounds.J Funct Biomater. 2012 Sep 5;3(3):601-14. doi: 10.3390/jfb3030601. J Funct Biomater. 2012. PMID: 24955635 Free PMC article.
-
Phosphatidylethanolamine-phosphatidylserine binding synergy of seven coagulation factors revealed using Nanodisc arrays on silicon photonic sensors.Sci Rep. 2020 Oct 15;10(1):17407. doi: 10.1038/s41598-020-73647-3. Sci Rep. 2020. PMID: 33060620 Free PMC article.
-
New organocatalyst scaffolds with high activity in promoting hydrazone and oxime formation at neutral pH.Org Lett. 2015 Jan 16;17(2):274-7. doi: 10.1021/ol503372j. Epub 2014 Dec 29. Org Lett. 2015. PMID: 25545888 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

