Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues
- PMID: 20809620
- PMCID: PMC2944909
- DOI: 10.1021/ja106284s
Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues
Abstract
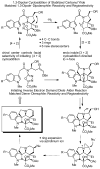
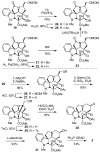
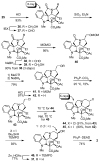
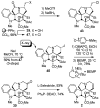
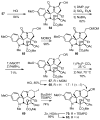
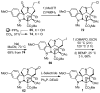
Concise asymmetric total syntheses of vindoline (1) and vindorosine (2) are detailed based on a unique intramolecular [4 + 2]/[3 + 2] cycloaddition cascade of 1,3,4-oxadiazoles inspired by the natural product structures. A chiral substituent on the tether linking the dienophile and oxadiazole was used to control the facial selectivity of the initiating Diels-Alder reaction and set the absolute stereochemistry of the remaining six stereocenters in the cascade cycloadduct. This key reaction introduced three rings and four C-C bonds central to the pentacyclic ring system setting all six stereocenters and introducing essentially all the functionality found in the natural products in a single step. Implementation of the approach for the synthesis of 1 and 2 required the development of a ring expansion reaction to provide a 6-membered ring suitably functionalized for introduction of the Δ(6,7)-double bond found in the core structure of the natural products. Two unique approaches were developed that defined our use of a protected hydroxymethyl group as the substituent that controls the stereochemical course of the cycloaddition cascade. In the course of these studies, several analogues of vindoline were prepared containing deep-seated structural changes presently accessible only by total synthesis. These analogues, bearing key modifications at C6-C8, were incorporated into vinblastine analogues and used to probe the unusual importance (100-fold) and define the potential role of the vinblastine Δ(6,7)-double bond.
Figures
Similar articles
-
Asymmetric total synthesis of vindoline.J Am Chem Soc. 2010 Mar 24;132(11):3685-7. doi: 10.1021/ja910695e. J Am Chem Soc. 2010. PMID: 20187641 Free PMC article.
-
Tandem Intramolecular Diels-Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles: Initial Scope and Applications.Acc Chem Res. 2016 Feb 16;49(2):241-51. doi: 10.1021/acs.accounts.5b00510. Epub 2016 Jan 27. Acc Chem Res. 2016. PMID: 26813287 Free PMC article.
-
Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids.J Am Chem Soc. 2006 Aug 16;128(32):10596-612. doi: 10.1021/ja061256t. J Am Chem Soc. 2006. PMID: 16895428 Free PMC article.
-
Total synthesis of (+)-vinblastine: control of the stereochemistry at C18'.Chem Rec. 2010 Apr;10(2):101-18. doi: 10.1002/tcr.201090001. Chem Rec. 2010. PMID: 20394103 Review.
-
Total synthesis of vinblastine, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure-function properties.Acc Chem Res. 2015 Mar 17;48(3):653-62. doi: 10.1021/ar500400w. Epub 2015 Jan 14. Acc Chem Res. 2015. PMID: 25586069 Free PMC article. Review.
Cited by
-
Key analogs of a uniquely potent synthetic vinblastine that contain modifications of the C20' ethyl substituent.Bioorg Med Chem Lett. 2017 Jul 15;27(14):3055-3059. doi: 10.1016/j.bmcl.2017.05.058. Epub 2017 May 18. Bioorg Med Chem Lett. 2017. PMID: 28551101 Free PMC article.
-
Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20'-vinblastine analogues.Org Lett. 2012 Mar 16;14(6):1428-31. doi: 10.1021/ol300173v. Epub 2012 Feb 28. Org Lett. 2012. PMID: 22369097 Free PMC article.
-
10'-Fluorovinblastine and 10'-Fluorovincristine: Synthesis of a Key Series of Modified Vinca Alkaloids.ACS Med Chem Lett. 2011 Dec 8;2(12):948-952. doi: 10.1021/ml200236a. ACS Med Chem Lett. 2011. PMID: 22247789 Free PMC article.
-
Indole-Based Small Molecules as Potential Therapeutic Agents for the Treatment of Fibrosis.Front Pharmacol. 2022 Feb 16;13:845892. doi: 10.3389/fphar.2022.845892. eCollection 2022. Front Pharmacol. 2022. PMID: 35250597 Free PMC article. Review.
-
A concise and versatile double-cyclization strategy for the highly stereoselective synthesis and arylative dimerization of Aspidosperma alkaloids.Angew Chem Int Ed Engl. 2012 May 7;51(19):4572-6. doi: 10.1002/anie.201200387. Epub 2012 Mar 12. Angew Chem Int Ed Engl. 2012. PMID: 22411850 Free PMC article. No abstract available.
References
-
- Gorman M, Neuss N, Biemann K. J Am Chem Soc. 1962;84:1058.
-
- Moncrief JW, Lipscomb WN. J Am Chem Soc. 1965;84:4963. - PubMed
-
-
Noble RL, Beer CT, Cutts JH. Ann N Y Acad Sci. 1958;76:882.Noble RL. Lloydia. 1964;27:280.Svoboda GH, Nuess N, Gorman M. J Am Pharm Assoc Sci Ed. 1959;48:659.For related, naturally occurring vinca alkaloids: see Leurosidine: Svoboda GH. Lloydia. 1961;24:173.Deoxyvinblastine: De Bruyn A, Sleechecker J, De Jonghe JP, Hannart J. Bull Soc Chim Belg. 1983;92:485.Neuss N, Gorman M, Cone NJ, Huckstep LL. Tetrahedron Lett. 1968:783.N-Desmethylvinblastine: Simonds R, De Bruyn A, De Taeye L, Verzele M, De Pauw C. Planta Med. 1984;50:274.Desacetoxyvinblastine: Neuss N, Barnes AJ, Jr, Huckstep LL. Experientia. 1975;31:18.Desacetylvinblastine: Svoboda GH, Barnes AJ., Jr J Pharm Sci. 1964;53:1227.Anhydrovinblastine: Goodbody AE, Watson CD, Chapple CCS, Vukovic J, Misawa M. Phytochem. 1988;27:1713.
-
-
-
Review: Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 229.
-
-
-
Reviews: Pearce HL. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 145.Borman LS, Kuehne ME. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 133.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources