Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum
- PMID: 20809939
- PMCID: PMC2942860
- DOI: 10.1186/1749-8104-5-23
Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum
Abstract
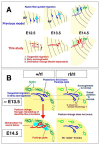
Background: Cerebellar corticogenesis begins with the assembly of Purkinje cells into the Purkinje plate (PP) by embryonic day 14.5 (E14.5) in mice. Although the dependence of PP formation on the secreted protein Reelin is well known and a prevailing model suggests that Purkinje cells migrate along the 'radial glial' fibers connecting the ventricular and pial surfaces, it is not clear how Purkinje cells behave in response to Reelin to initiate the PP. Furthermore, it is not known what nascent Purkinje cells look like in vivo. When and how Purkinje cells start axonogenesis must also be elucidated.
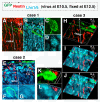
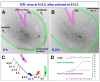
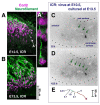
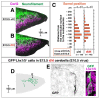
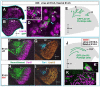
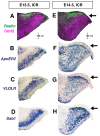
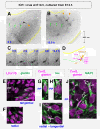
Results: We show that Purkinje cells generated on E10.5 in the posterior periventricular region of the lateral cerebellum migrate tangentially, after only transiently migrating radially, towards the anterior, exhibiting an elongated morphology consistent with axonogenesis at E12.5. After their somata reach the outer/dorsal region by E13.5, they change 'posture' by E14.5 through remodeling of non-axon (dendrite-like) processes and a switchback-like mode of somal movement towards a superficial Reelin-rich zone, while their axon-like fibers remain relatively deep, which demarcates the somata-packed portion as a plate. In reeler cerebella, the early born posterior lateral Purkinje cells are initially normal during migration with anteriorly extended axon-like fibers until E13.5, but then fail to form the PP due to lack of the posture-change step.
Conclusions: Previously unknown behaviors are revealed for a subset of Purkinje cells born early in the posteior lateral cerebellum: tangential migration; early axonogenesis; and Reelin-dependent reorientation initiating PP formation. This study provides a solid basis for further elucidation of Reelin's function and the mechanisms underlying the cerebellar corticogenesis, and will contribute to the understanding of how polarization of individual cells drives overall brain morphogenesis.
Figures




Similar articles
-
Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody.J Neurosci. 1997 May 15;17(10):3599-609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. J Neurosci. 1997. PMID: 9133383 Free PMC article.
-
Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages.J Neurosci. 2009 Aug 26;29(34):10653-62. doi: 10.1523/JNEUROSCI.0345-09.2009. J Neurosci. 2009. PMID: 19710317 Free PMC article.
-
Role of Reelin in cell positioning in the cerebellum and the cerebellum-like structure in zebrafish.Dev Biol. 2019 Nov 15;455(2):393-408. doi: 10.1016/j.ydbio.2019.07.010. Epub 2019 Jul 16. Dev Biol. 2019. PMID: 31323192
-
[Cytoarchitectonic abnormality in the facial nucleus of the reeler mouse].Kaibogaku Zasshi. 1999 Aug;74(4):411-20. Kaibogaku Zasshi. 1999. PMID: 10496086 Review. Japanese.
-
Role of Reelin in the development and maintenance of cortical lamination.J Neural Transm (Vienna). 2009 Nov;116(11):1451-5. doi: 10.1007/s00702-009-0228-7. Epub 2009 Apr 25. J Neural Transm (Vienna). 2009. PMID: 19396394 Review.
Cited by
-
Genetic and Molecular Approaches to Study Neuronal Migration in the Developing Cerebral Cortex.Brain Sci. 2017 May 5;7(5):53. doi: 10.3390/brainsci7050053. Brain Sci. 2017. PMID: 28475113 Free PMC article. Review.
-
Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation.Biomolecules. 2020 Jun 26;10(6):964. doi: 10.3390/biom10060964. Biomolecules. 2020. PMID: 32604886 Free PMC article. Review.
-
CORL Expression and Function in Insulin Producing Neurons Reversibly Influences Adult Longevity in Drosophila.G3 (Bethesda). 2018 Aug 30;8(9):2979-2990. doi: 10.1534/g3.118.200572. G3 (Bethesda). 2018. PMID: 30006413 Free PMC article.
-
Diagnostic Approach to Cerebellar Hypoplasia.Cerebellum. 2021 Aug;20(4):631-658. doi: 10.1007/s12311-020-01224-5. Epub 2021 Feb 3. Cerebellum. 2021. PMID: 33534089 Review.
-
Cerebellum Lecture: the Cerebellar Nuclei-Core of the Cerebellum.Cerebellum. 2024 Apr;23(2):620-677. doi: 10.1007/s12311-022-01506-0. Epub 2023 Feb 13. Cerebellum. 2024. PMID: 36781689 Free PMC article. Review.
References
-
- Altman J, Bayer SA. Development of the Cerebellar System in Relation to its Evolution, Structure, and Functions. Boca Raton: CRC press; 1997.
-
- Hoshino M, Nakamura S, Mori K, Kawuchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

