The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens
- PMID: 20809975
- PMCID: PMC2940857
- DOI: 10.1186/1471-2180-10-232
The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens
Abstract
Background: Poultry meat is one of the most important sources of human campylobacteriosis, an acute bacterial enteritis which is a major problem worldwide. Campylobacter coli and Campylobacter jejuni are the most common Campylobacter species associated with this disease. These pathogens live in the intestinal tract of most avian species and under commercial conditions they spread rapidly to infect a high proportion of the flock, which makes their treatment and prevention very difficult. Bacteriophages (phages) are naturally occurring predators of bacteria with high specificity and also the capacity to evolve to overcome bacterial resistance. Therefore phage therapy is a promising alternative to antibiotics in animal production. This study tested the efficacy of a phage cocktail composed of three phages for the control of poultry infected with C. coli and C. jejuni. Moreover, it evaluated the effectiveness of two routes of phage administration (by oral gavage and in feed) in order to provide additional information regarding their future use in a poultry unit.
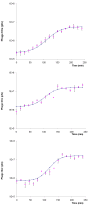
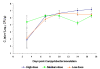
Results: The results indicate that experimental colonisation of chicks was successful and that the birds showed no signs of disease even at the highest dose of Campylobacter administered. The phage cocktail was able to reduce the titre of both C. coli and C. jejuni in faeces by approximately 2 log10 cfu/g when administered by oral gavage and in feed. This reduction persisted throughout the experimental period and neither pathogen regained their former numbers. The reduction in Campylobacter titre was achieved earlier (2 days post-phage administration) when the phage cocktail was incorporated in the birds' feed. Campylobacter strains resistant to phage infection were recovered from phage-treated chickens at a frequency of 13%. These resistant phenotypes did not exhibit a reduced ability to colonize the chicken guts and did not revert to sensitive types.
Conclusions: Our findings provide further evidence of the efficacy of phage therapy for the control of Campylobacter in poultry. The broad host range of the novel phage cocktail enabled it to target both C. jejuni and C. coli strains. Moreover the reduction of Campylobacter by approximately 2 log10cfu/g, as occurred in our study, could lead to a 30-fold reduction in the incidence of campylobacteriosis associated with consumption of chicken meals (according to mathematical models). To our knowledge this is the first report of phage being administered in feed to Campylobacter-infected chicks and our results show that it lead to an earlier and more sustainable reduction of Campylobacter than administration by oral gavage. Therefore the present study is of extreme importance as it has shown that administering phages to poultry via the food could be successful on a commercial scale.
Figures

Similar articles
-
Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens.J Food Prot. 2009 Apr;72(4):733-40. doi: 10.4315/0362-028x-72.4.733. J Food Prot. 2009. PMID: 19435220
-
Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens.Appl Environ Microbiol. 2005 Nov;71(11):6554-63. doi: 10.1128/AEM.71.11.6554-6563.2005. Appl Environ Microbiol. 2005. PMID: 16269681 Free PMC article.
-
Reduction of Campylobacter jejuni in broiler chicken by successive application of group II and group III phages.PLoS One. 2014 Dec 9;9(12):e114785. doi: 10.1371/journal.pone.0114785. eCollection 2014. PLoS One. 2014. PMID: 25490713 Free PMC article.
-
The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms.Appl Environ Microbiol. 2017 May 31;83(12):e00416-17. doi: 10.1128/AEM.00416-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411226 Free PMC article. Review.
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
Cited by
-
Phage Therapy in Veterinary Medicine.Antibiotics (Basel). 2021 Apr 11;10(4):421. doi: 10.3390/antibiotics10040421. Antibiotics (Basel). 2021. PMID: 33920369 Free PMC article. Review.
-
Bacteriophages as Biocontrol Agents in Livestock Food Production.Microorganisms. 2022 Oct 27;10(11):2126. doi: 10.3390/microorganisms10112126. Microorganisms. 2022. PMID: 36363718 Free PMC article. Review.
-
Campylobacter group II phage CP21 is the prototype of a new subgroup revealing a distinct modular genome organization and host specificity.BMC Genomics. 2015 Aug 22;16(1):629. doi: 10.1186/s12864-015-1837-1. BMC Genomics. 2015. PMID: 26296758 Free PMC article.
-
Global Epidemiology of Campylobacter Infection.Clin Microbiol Rev. 2015 Jul;28(3):687-720. doi: 10.1128/CMR.00006-15. Clin Microbiol Rev. 2015. PMID: 26062576 Free PMC article. Review.
-
Campylobacter Bacteriophage Cocktail Design Based on an Advanced Selection Scheme.Antibiotics (Basel). 2022 Feb 10;11(2):228. doi: 10.3390/antibiotics11020228. Antibiotics (Basel). 2022. PMID: 35203830 Free PMC article.
References
-
- Friedman C, Neimann J, Wegener H, Tauxe R. In: Campylobacter. 2. Nachamkin I, Blaser MJ, editor. Washington D.C. ASM Press; 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations; pp. 121–138.
-
- Lindqvist R, Andersson Y, Lindback J, Wegscheider M, Eriksson Y, Tidestrom L, Lagerqvist-Widh A, Hedlund KO, Lofdahl S, Svensson L, Norinder A. A one-year study of foodborne illnesses in the municipality of Uppsala, Sweden. Emerg Infect Dis. 2001;7:588–592. doi: 10.3201/eid0703.010342. - DOI - PMC - PubMed
-
- Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, McGivern T, Kassenborg H, Reilly K, Kennedy M, Angulo F, Tauxe RV. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999. Clin Infect Dis. 2004;38(Suppl 3):S165–174. doi: 10.1086/381583. - DOI - PubMed
-
- Jacobs-Reitsma W. In: Campylobacter. 2. Nachamkin I, Blaser MJ, editor. Washington D.C. ASM Press; 2000. Campylobacter in the food supply; pp. 467–481.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

