Targeting the chromosome partitioning protein ParA in tuberculosis drug discovery
- PMID: 20810423
- PMCID: PMC2980951
- DOI: 10.1093/jac/dkq311
Targeting the chromosome partitioning protein ParA in tuberculosis drug discovery
Abstract
Objective: To identify inhibitors of the essential chromosome partitioning protein ParA that are active against Mycobacterium tuberculosis.
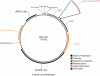
Methods: Antisense expression of the parA orthologue MSMEG_6939 was induced on the Mycobacterium smegmatis background. Screening of synthetic chemical libraries was performed to identify compounds with higher anti-mycobacterial activity in the presence of parA antisense. Differentially active compounds were validated for specific inhibition of purified ParA protein from M. tuberculosis (Rv3918c). ParA inhibitors were then characterized for their activity towards M. tuberculosis in vitro.
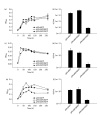
Results: Under a number of culture conditions, parA antisense expression in M. smegmatis resulted in reduced growth. This effect on growth provided a basis for the detection of compounds that increased susceptibility to expression of parA antisense. Two compounds identified from library screening, phenoxybenzamine and octoclothepin, also inhibited the in vitro ATPase activity of ParA from M. tuberculosis. Structural in silico analyses predict that phenoxybenzamine and octoclothepin undergo interactions compatible with the active site of ParA. Octoclothepin exhibited significant bacteriostatic activity towards M. tuberculosis.
Conclusions: Our data support the use of whole-cell differential antisense screens for the discovery of inhibitors of specific anti-tubercular drug targets. Using this approach, we have identified an inhibitor of purified ParA and whole cells of M. tuberculosis.
Figures




References
-
- Berman HM, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. doi:10.1093/nar/28.1.235. - DOI - PMC - PubMed
-
- Hershkovitz I, Donoghue HD, Minnikin DE, et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS ONE. 2008;3:e3426. doi:10.1371/journal.pone.0003426. - DOI - PMC - PubMed
-
- WHO. World Health Organization Report 2009. Global Tuberculosis Control: Epidemiology; Strategy; Financing. http://www.who.int/tb/publications/global_report/2009/pdf/full_report.pdf. (10 August 2010, date last accessed)
-
- IUATLD. International Union Against Tuberculosis and Lung Disease, Anti-Tuberculosis Drug Resistance in the World, Report No. 4. 2008.
-
- WHO. World Health Organization and the Stop TB Partnership. Building on and Enhancing DOTS to Meet the TB-Related Millennium Development Goals. http://www.who.int/tb/publications/2006/stop_tb_strategy.pdf. (10 August 2010, date last accessed)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

