Characterization of mesonephric development and regeneration using transgenic zebrafish
- PMID: 20810610
- PMCID: PMC2980409
- DOI: 10.1152/ajprenal.00394.2010
Characterization of mesonephric development and regeneration using transgenic zebrafish
Abstract
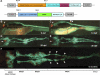
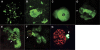
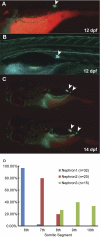
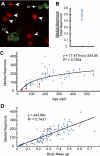
The zebrafish is a valuable vertebrate model for kidney research. The majority of previous studies focused on the zebrafish pronephros, which comprises only two nephrons and is structurally simpler than the mesonephros of adult fish and the metanephros of mammals. To evaluate the zebrafish system for more complex studies of kidney development and regeneration, we investigated the development and postinjury regeneration of the mesonephros in adult zebrafish. Utilizing two transgenic zebrafish lines (wt1b::GFP and pod::NTR-mCherry), we characterized the developmental stages of individual mesonephric nephrons and the temporal-spatial pattern of mesonephrogenesis. We found that mesonephrogenesis continues throughout the life of zebrafish, with a rapid growth phase during the juvenile period and a slower phase in adulthood such that the total nephron number of juvenile and adult fish linearly correlates with body mass. Following gentamicin-induced renal injury, the zebrafish mesonephros can undergo de novo regeneration of mesonephric nephrons, a process known as neonephrogenesis. We found that wt1b expression was induced in individually dispersed cells in the mesonephric interstitium as early as 48 h following injury. These wt1b-expressing cells formed aggregates by 72-96 h following injury which proceeded to form nephrons. This suggests that wt1b may serve as an early marker of fated renal progenitor cells. The synchronous nature of regenerative neonephrogenesis suggests that this process may be useful for studies of nephron development.
Figures

Similar articles
-
Kidney Regeneration in Adult Zebrafish by Gentamicin Induced Injury.J Vis Exp. 2015 Aug 3;(102):e51912. doi: 10.3791/51912. J Vis Exp. 2015. PMID: 26275011 Free PMC article.
-
Identification of adult nephron progenitors capable of kidney regeneration in zebrafish.Nature. 2011 Feb 3;470(7332):95-100. doi: 10.1038/nature09669. Epub 2011 Jan 26. Nature. 2011. PMID: 21270795 Free PMC article.
-
Development of the zebrafish mesonephros.Genesis. 2015 Mar-Apr;53(3-4):257-69. doi: 10.1002/dvg.22846. Epub 2015 Mar 14. Genesis. 2015. PMID: 25677367 Free PMC article.
-
Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration.Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):559-85. doi: 10.1002/wdev.92. Epub 2012 Oct 16. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014448 Free PMC article. Review.
-
Kidney injury and regeneration in zebrafish.Semin Nephrol. 2014 Jul;34(4):437-44. doi: 10.1016/j.semnephrol.2014.06.010. Epub 2014 Jun 13. Semin Nephrol. 2014. PMID: 25217272 Review.
Cited by
-
Modeling Podocyte Ontogeny and Podocytopathies with the Zebrafish.J Dev Biol. 2023 Feb 20;11(1):9. doi: 10.3390/jdb11010009. J Dev Biol. 2023. PMID: 36810461 Free PMC article. Review.
-
Temporal and spatial expression of tight junction genes during zebrafish pronephros development.Gene Expr Patterns. 2014 Nov;16(2):104-13. doi: 10.1016/j.gep.2014.11.001. Epub 2014 Nov 7. Gene Expr Patterns. 2014. PMID: 25460834 Free PMC article.
-
Principles of Zebrafish Nephron Segment Development.J Dev Biol. 2023 Mar 18;11(1):14. doi: 10.3390/jdb11010014. J Dev Biol. 2023. PMID: 36976103 Free PMC article. Review.
-
Transgenic fluorescent zebrafish lines that have revolutionized biomedical research.Lab Anim Res. 2021 Sep 8;37(1):26. doi: 10.1186/s42826-021-00103-2. Lab Anim Res. 2021. PMID: 34496973 Free PMC article. Review.
-
(Zebra)fishing for nephrogenesis genes.Tissue Barriers. 2024 Apr 2;12(2):2219605. doi: 10.1080/21688370.2023.2219605. Epub 2023 May 31. Tissue Barriers. 2024. PMID: 37254823 Free PMC article. Review.
References
-
- Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn 235: 554–561, 2006 - PubMed
-
- Bollig F, Perner B, Besenbeck B, Kothe S, Ebert C, Taudien S, Englert C. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development 136: 2883–2892, 2009 - PubMed
-
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236: 1025–1035, 2007 - PubMed
-
- Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol 16: 299–304, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

