Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus
- PMID: 20810902
- PMCID: PMC6633430
- DOI: 10.1523/JNEUROSCI.2012-10.2010
Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus
Abstract
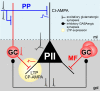
Fast-spiking perisomatic-inhibitory interneurons (PIIs) receive convergent excitation and mediate both feedforward and feedback inhibition in cortical microcircuits. However, it remains poorly understood how convergent excitatory inputs recruit PIIs to produce precisely timed inhibition. Here, we analyzed the interaction of inputs from the entorhinal cortex [perforant path (PP)] and from local granule cells [mossy fibers (MFs)] onto PIIs in the rat dentate gyrus (DG). PP stimulation alone activates PIIs with low temporal precision. Interestingly, when PP and MFs are coactivated with a 10 ms delay, PIIs discharge with precise timing. Moreover, repeated coactivation of the two inputs induces associative long-term potentiation (LTP) at MF synapses. Under these conditions, a single potentiated MF input is sufficient to recruit PIIs in a reliable and highly precise manner to provide feedback inhibition. MF-LTP depends on the discharge of PIIs, indicating Hebbian plasticity. However, MF-LTP is preserved when NMDA receptors are blocked but depends on transmission through Ca(2+)-permeable AMPA receptors (AMPARs). PP-PII synapses, in contrast, lack Ca(2+)-permeable AMPARs and do not show plasticity on associative activation. Thus, precise recruitment of PIIs requires excitation through MF-PII synapses during feedforward activation. We propose that associative plasticity at these synapses is a central mechanism that adjusts inhibition levels to maintain sparse activity and to improve signal-to-noise ratio in the DG network.
Figures



Similar articles
-
Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons.Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13211-6. doi: 10.1073/pnas.1409394111. Epub 2014 Aug 26. Proc Natl Acad Sci U S A. 2014. PMID: 25161282 Free PMC article.
-
Single Bursts of Individual Granule Cells Functionally Rearrange Feedforward Inhibition.J Neurosci. 2018 Feb 14;38(7):1711-1724. doi: 10.1523/JNEUROSCI.1595-17.2018. Epub 2018 Jan 15. J Neurosci. 2018. PMID: 29335356 Free PMC article.
-
Plasticity of NMDA receptor-mediated excitatory postsynaptic currents at perforant path inputs to dendrite-targeting interneurons.J Physiol. 2012 Aug 15;590(16):3771-86. doi: 10.1113/jphysiol.2012.234740. Epub 2012 May 21. J Physiol. 2012. PMID: 22615437 Free PMC article.
-
Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons.Neuropharmacology. 2011 Apr;60(5):740-7. doi: 10.1016/j.neuropharm.2010.11.008. Epub 2010 Nov 18. Neuropharmacology. 2011. PMID: 21093459 Free PMC article. Review.
-
Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity.Neuropharmacology. 2011 Apr;60(5):730-9. doi: 10.1016/j.neuropharm.2010.12.017. Epub 2010 Dec 30. Neuropharmacology. 2011. PMID: 21195097 Review.
Cited by
-
Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons.Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13211-6. doi: 10.1073/pnas.1409394111. Epub 2014 Aug 26. Proc Natl Acad Sci U S A. 2014. PMID: 25161282 Free PMC article.
-
Quantitative properties of a feedback circuit predict frequency-dependent pattern separation.Elife. 2020 Feb 20;9:e53148. doi: 10.7554/eLife.53148. Elife. 2020. PMID: 32077850 Free PMC article.
-
Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation.J Neurosci. 2015 Mar 11;35(10):4131-9. doi: 10.1523/JNEUROSCI.3671-14.2015. J Neurosci. 2015. PMID: 25762660 Free PMC article.
-
Endogenous zinc depresses GABAergic transmission via T-type Ca(2+) channels and broadens the time window for integration of glutamatergic inputs in dentate granule cells.J Physiol. 2014 Jan 1;592(1):67-86. doi: 10.1113/jphysiol.2013.261420. Epub 2013 Sep 30. J Physiol. 2014. PMID: 24081159 Free PMC article.
-
Functional properties of GABA synaptic inputs onto GABA neurons in monkey prefrontal cortex.J Neurophysiol. 2015 Mar 15;113(6):1850-61. doi: 10.1152/jn.00799.2014. Epub 2014 Dec 24. J Neurophysiol. 2015. PMID: 25540225 Free PMC article.
References
-
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–234. - PubMed
-
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 1999;82:1295–12302. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
