Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme
- PMID: 20810904
- PMCID: PMC6633413
- DOI: 10.1523/JNEUROSCI.2985-10.2010
Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme
Abstract
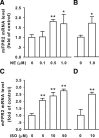
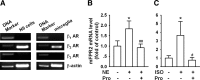
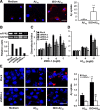
Locus ceruleus (LC) is the main subcortical site of norepinephrine synthesis. In Alzheimer's disease (AD) patients and rodent models, degeneration of LC neurons and reduced levels of norepinephrine in LC projection areas are significantly correlated with the increase in amyloid plaques, neurofibrillary tangles, and severity of dementia. Activated microglia play a pivotal role in the progression of AD by either clearing amyloid beta peptide (Abeta) deposits through uptake of Abeta or releasing cytotoxic substances and proinflammatory cytokines. Here, we investigated the effect of norepinephrine on Abeta uptake and clearance by murine microglia and explored the underlying mechanisms. We found that murine microglia cell line N9 and primary microglia expressed beta(2) adrenergic receptor (AR) but not beta(1) and beta(3)AR. Norepinephrine and isoproterenol upregulated the expression of Abeta receptor mFPR2, a mouse homolog of human formyl peptide receptor FPR2, through activation of beta(2)AR in microglia. Norepinephrine also induced mFPR2 expression in mouse brain. Activation of beta(2)AR in microglia promoted Abeta(42) uptake through upregulation of mFPR2 and enhanced spontaneous cell migration but had no effect on cell migration in response to mFPR2 agonists. Furthermore, activation of beta(2)AR on microglia induced the expression of insulin-degrading enzyme and increased the degradation of Abeta(42). Mechanistic studies showed that isoproterenol induced mFPR2 expression through ERK1/2-NF-kappaB and p38-NF-kappaB signaling pathways. These findings suggest that noradrenergic innervation from LC is needed to maintain adequate Abeta uptake and clearance by microglia, and norepinephrine is a link between neuron and microglia to orchestrate the host response to Abeta in AD.
Figures




Similar articles
-
Androgen alleviates neurotoxicity of β-amyloid peptide (Aβ) by promoting microglial clearance of Aβ and inhibiting microglial inflammatory response to Aβ.CNS Neurosci Ther. 2017 Nov;23(11):855-865. doi: 10.1111/cns.12757. Epub 2017 Sep 20. CNS Neurosci Ther. 2017. PMID: 28941188 Free PMC article.
-
CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid beta 1-42 peptide by up-regulating the expression of the G-protein- coupled receptor mFPR2.FASEB J. 2005 Dec;19(14):2032-4. doi: 10.1096/fj.05-4578fje. Epub 2005 Oct 11. FASEB J. 2005. PMID: 16219804
-
IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia.J Immunol. 2008 Nov 1;181(9):6503-13. doi: 10.4049/jimmunol.181.9.6503. J Immunol. 2008. PMID: 18941241
-
Microglia receptors and their implications in the response to amyloid β for Alzheimer's disease pathogenesis.J Neuroinflammation. 2014 Mar 13;11:48. doi: 10.1186/1742-2094-11-48. J Neuroinflammation. 2014. PMID: 24625061 Free PMC article. Review.
-
Microglial Aβ receptors in Alzheimer's disease.Cell Mol Neurobiol. 2015 Jan;35(1):71-83. doi: 10.1007/s10571-014-0101-6. Epub 2014 Aug 23. Cell Mol Neurobiol. 2015. PMID: 25149075 Free PMC article. Review.
Cited by
-
Role of catalpol in ameliorating the pathogenesis of experimental autoimmune encephalomyelitis by increasing the level of noradrenaline in the locus coeruleus.Mol Med Rep. 2018 Mar;17(3):4163-4172. doi: 10.3892/mmr.2018.8378. Epub 2018 Jan 5. Mol Med Rep. 2018. PMID: 29328415 Free PMC article.
-
Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs.Mol Neurobiol. 2018 Aug;55(8):6894-6905. doi: 10.1007/s12035-018-0889-z. Epub 2018 Jan 22. Mol Neurobiol. 2018. PMID: 29357041
-
Brain plasticity-based therapeutics.Front Hum Neurosci. 2014 Jun 27;8:385. doi: 10.3389/fnhum.2014.00385. eCollection 2014. Front Hum Neurosci. 2014. PMID: 25018719 Free PMC article. Review.
-
Neuroprotective roles of the P2Y(2) receptor.Purinergic Signal. 2012 Sep;8(3):559-78. doi: 10.1007/s11302-012-9307-6. Epub 2012 Apr 14. Purinergic Signal. 2012. PMID: 22528682 Free PMC article. Review.
-
Perspectives in molecular imaging using staging biomarkers and immunotherapies in Alzheimer's disease.ScientificWorldJournal. 2013;2013:589308. doi: 10.1155/2013/589308. Epub 2013 Feb 5. ScientificWorldJournal. 2013. PMID: 23476143 Free PMC article. Review.
References
-
- Aloisi F, Ambrosini E, Columba-Cabezas S, Magliozzi R, Serafini B. Intracerebral regulation of immune responses. Ann Med. 2001;33:510–515. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. - PubMed
-
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, Hauser DL. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–262. - PubMed
-
- Carnevale D, De Simone R, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6:388–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
