PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions
- PMID: 20810911
- PMCID: PMC3006334
- DOI: 10.1152/ajpcell.00246.2010
PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions
Abstract
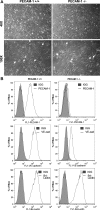
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin superfamily of cell adhesion molecules with important roles in angiogenesis and inflammation. However, the molecular and cellular mechanisms, and the role that specific PECAM-1 isoforms play in these processes, remain elusive. We recently showed attenuation of retinal vascular development and neovascularization in PECAM-1-deficient (PECAM-1-/-) mice. To gain further insight into the role of PECAM-1 in these processes, we isolated primary retinal endothelial cells (EC) from wild-type (PECAM-1+/+) and PECAM-1-/- mice. Lack of PECAM-1 had a significant impact on endothelial cell-cell and cell-matrix interactions, resulting in attenuation of cell migration and capillary morphogenesis. Mechanistically these changes were associated with a significant decrease in expression of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) bioavailability in PECAM-1-/- retinal EC. PECAM-1-/- retinal EC also exhibited a lower rate of apoptosis under basal and challenged conditions, consistent with their increased growth rate. Furthermore, reexpression of PECAM-1 was sufficient to restore migration and capillary morphogenesis of null cells in an isoform-specific manner. Thus PECAM-1 expression modulates proangiogenic properties of EC, and these activities are significantly influenced by alternative splicing of its cytoplasmic domain.
Figures








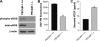
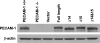


References
-
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol 25: 1590– 1595, 2005 - PubMed
-
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147– 157, 1999 - PubMed
-
- Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27: 6285– 6298, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

