Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease
- PMID: 20810998
- PMCID: PMC3013493
- DOI: 10.1212/WNL.0b013e3181f6128c
Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease
Abstract
Objective: To investigate if a central cholinesterase inhibitor will reduce falling frequency in subjects with Parkinson disease (PD) with advanced postural instability.
Background: Falling due to postural instability is a significant problem in advancing PD, and is minimally impacted by dopaminergic therapy. Anticholinergic medications increase falling in the elderly. Further, CNS cholinergic neuron loss occurs in PD. We hypothesized that acetylcholine augmentation may reduce frequent falling in subjects with PD.
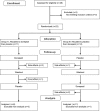
Methods: We enrolled 23 subjects with PD who reported falling or nearly falling more than 2 times per week. In a randomized, placebo-controlled, crossover design, subjects were given 6 weeks of donepezil or placebo with a 3-week washout between phases. The primary outcomes were daily falls and near falls reported on postcards. Secondary outcomes included scores on the Activities of Balance Confidence Scale, Berg Balance Scale, Clinical Global Impression of Change, Folstein Mini-Mental State Examination, and the motor section of the Unified Parkinson's Disease Rating Scale.
Results: Fall frequency per day on placebo was 0.25 ± 0.08 (SEM) compared with 0.13 ± 0.03 on donepezil (p < 0.05). The frequency of near falls was not significantly different between phases. The secondary outcomes did not differ; however, there was a trend to improvement on the subject-completed Global Impression of Change scale.
Conclusions: Subjects with PD fell approximately half as often during the 6 weeks on donepezil than on placebo. Larger trials of cholinergic augmentation are warranted in subjects with PD with frequent falls.
Classification of evidence: This study provides Class II evidence that donepezil (maximum 10 mg per day) significantly reduced the number of falls in patients with PD (0.13 falls/day, SEM = 0.03) than when taking placebo (0.25 falls/day, SEM = 0.08, p = 0.049).
Figures
Comment in
-
Think before you leap: donepezil reduces falls?Neurology. 2010 Oct 5;75(14):1226-7. doi: 10.1212/WNL.0b013e3181f5d507. Epub 2010 Sep 8. Neurology. 2010. PMID: 20826715 No abstract available.
References
-
- Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson's disease: characteristics of fallers and non-fallers. Age Ageing 2001;30:47–52. - PubMed
-
- Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs 2000;32:222–228. - PubMed
-
- Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord 2003;18:496–502. - PubMed
-
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol 2001;248:950–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials