Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice
- PMID: 20811044
- PMCID: PMC3053271
- DOI: 10.1167/iovs.10-6113
Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice
Abstract
Purpose: Iron dysregulation can cause retinal disease, yet retinal iron regulatory mechanisms are incompletely understood. The peptide hormone hepcidin (Hepc) limits iron uptake from the intestine by triggering degradation of the iron transporter ferroportin (Fpn). Given that Hepc is expressed in the retina and Fpn is expressed in cells constituting the blood-retinal barrier, the authors tested whether the retina may produce Hepc to limit retinal iron import.
Methods: Retinas of Hepc(-/-) mice were analyzed by histology, autofluorescence spectral analysis, atomic absorption spectrophotometry, Perls' iron stain, and immunofluorescence to assess iron-handling proteins. Retinal Hepc mRNA was evaluated through qPCR after intravitreal iron injection. Mechanisms of retinal Hepc upregulation were tested by Western blot analysis. A retinal capillary endothelial cell culture system was used to assess the effect of exogenous Hepc on Fpn.
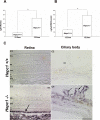
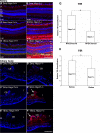
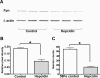
Results: Hepc(-/-) mice experienced age-dependent increases in retinal iron followed by retinal degeneration with autofluorescent RPE, photoreceptor death, and subretinal neovascularization. Hepc(-/-) mice had increased Fpn immunoreactivity in vascular endothelial cells. Conversely, in cultured retinal capillary endothelial cells, exogenous Hepc decreased both Fpn levels and iron transport. The retina can sense increased iron levels, upregulating Hepc after phosphorylation of extracellular signal regulated kinases.
Conclusions: These findings indicate that Hepc is essential for retinal iron regulation. In the absence of Hepc, retinal degeneration occurs. Increases in Hepc mRNA levels after intravitreal iron injection combined with Hepc-mediated decreases in iron export from cultured retinal capillary endothelial cells suggest that the retina may use Hepc for its tissue-specific iron regulation.
Figures





Similar articles
-
The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice.Invest Ophthalmol Vis Sci. 2014 Jun 26;55(7):4525-32. doi: 10.1167/iovs.14-14568. Invest Ophthalmol Vis Sci. 2014. PMID: 24970260 Free PMC article.
-
Mice with hepcidin-resistant ferroportin accumulate iron in the retina.FASEB J. 2016 Feb;30(2):813-23. doi: 10.1096/fj.15-276758. Epub 2015 Oct 27. FASEB J. 2016. PMID: 26506980 Free PMC article.
-
Liver-Specific, but Not Retina-Specific, Hepcidin Knockout Causes Retinal Iron Accumulation and Degeneration.Am J Pathol. 2019 Sep;189(9):1814-1830. doi: 10.1016/j.ajpath.2019.05.022. Epub 2019 Jul 6. Am J Pathol. 2019. PMID: 31287995 Free PMC article.
-
Hepcidin and ferroportin: the new players in iron metabolism.Semin Liver Dis. 2011 Aug;31(3):272-9. doi: 10.1055/s-0031-1286058. Epub 2011 Sep 7. Semin Liver Dis. 2011. PMID: 21901657 Free PMC article. Review.
-
Expression and function of iron-regulatory proteins in retina.IUBMB Life. 2010 May;62(5):363-70. doi: 10.1002/iub.326. IUBMB Life. 2010. PMID: 20408179 Free PMC article. Review.
Cited by
-
Reducing iron accumulation: A potential approach for the prevention and treatment of postmenopausal osteoporosis.Exp Ther Med. 2015 Jul;10(1):7-11. doi: 10.3892/etm.2015.2484. Epub 2015 May 8. Exp Ther Med. 2015. PMID: 26170904 Free PMC article.
-
Retinal expression of the serine protease matriptase-2 (Tmprss6) and its role in retinal iron homeostasis.Mol Vis. 2014 Apr 26;20:561-74. eCollection 2014. Mol Vis. 2014. PMID: 24791141 Free PMC article.
-
Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells.PLoS One. 2014 Feb 12;9(2):e89003. doi: 10.1371/journal.pone.0089003. eCollection 2014. PLoS One. 2014. PMID: 24533165 Free PMC article.
-
Inducible RPE-specific GPX4 knockout causes oxidative stress and retinal degeneration with features of age-related macular degeneration.Exp Eye Res. 2024 Oct;247:110028. doi: 10.1016/j.exer.2024.110028. Epub 2024 Aug 10. Exp Eye Res. 2024. PMID: 39128667
-
Ferritin overexpression in Drosophila glia leads to iron deposition in the optic lobes and late-onset behavioral defects.Neurobiol Dis. 2011 Jul;43(1):213-9. doi: 10.1016/j.nbd.2011.03.013. Epub 2011 Apr 1. Neurobiol Dis. 2011. PMID: 21440626 Free PMC article.
References
-
- Hahn P, Ying GS, Beard J, Dunaief JL. Iron levels in human retina: sex difference and increase with age. Neuroreport. 2006;17:1803–1806 - PubMed
-
- Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta. 2009;1790:718–723 - PubMed
-
- Weinberg ED. Iron out-of-balance: a risk factor for acute and chronic diseases. Hemoglobin. 2008;32:117–122 - PubMed
-
- Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

