Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies
- PMID: 20811160
- PMCID: PMC2929718
- DOI: 10.1172/JCI42258
Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies
Erratum in
- J Clin Invest. 2011 Jan 4;121(1):456-7
Abstract
Nonsyndromic recessive retinal dystrophies cause severe visual impairment due to the death of photoreceptor and retinal pigment epithelium cells. These diseases until recently have been considered to be incurable. Molecular genetic studies in the last two decades have revealed the underlying molecular causes in approximately two-thirds of patients. The mammalian eye has been at the forefront of therapeutic trials based on gene augmentation in humans with an early-onset nonsyndromic recessive retinal dystrophy due to mutations in the retinal pigment epithelium-specific protein 65kDa (RPE65) gene. Tremendous challenges still lie ahead to extrapolate these studies to other retinal disease-causing genes, as human gene augmentation studies require testing in animal models for each individual gene and sufficiently large patient cohorts for clinical trials remain to be identified through cost-effective mutation screening protocols.
Figures
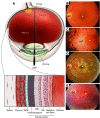




References
-
- Heckenlively J.Retinitis Pigmentosa . New York, New York, USA: Lippincott Williams & Wilkins; 1988.
-
- Blacharski PA. Fundus flavimaculatus. In: Newsome DA, ed.Retinal Dystrophies And Degenerations . New York, New York, USA: Raven Press; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

