Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease
- PMID: 20811458
- PMCID: PMC2936959
- DOI: 10.1038/nature09325
Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease
Abstract
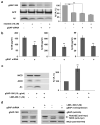
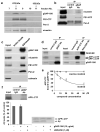
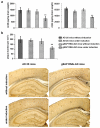
Accumulation of neurotoxic amyloid-beta is a major hallmark of Alzheimer's disease. Formation of amyloid-beta is catalysed by gamma-secretase, a protease with numerous substrates. Little is known about the molecular mechanisms that confer substrate specificity on this potentially promiscuous enzyme. Knowledge of the mechanisms underlying its selectivity is critical for the development of clinically effective gamma-secretase inhibitors that can reduce amyloid-beta formation without impairing cleavage of other gamma-secretase substrates, especially Notch, which is essential for normal biological functions. Here we report the discovery of a novel gamma-secretase activating protein (GSAP) that drastically and selectively increases amyloid-beta production through a mechanism involving its interactions with both gamma-secretase and its substrate, the amyloid precursor protein carboxy-terminal fragment (APP-CTF). GSAP does not interact with Notch, nor does it affect its cleavage. Recombinant GSAP stimulates amyloid-beta production in vitro. Reducing GSAP concentrations in cell lines decreases amyloid-beta concentrations. Knockdown of GSAP in a mouse model of Alzheimer's disease reduces levels of amyloid-beta and plaque development. GSAP represents a type of gamma-secretase regulator that directs enzyme specificity by interacting with a specific substrate. We demonstrate that imatinib, an anticancer drug previously found to inhibit amyloid-beta formation without affecting Notch cleavage, achieves its amyloid-beta-lowering effect by preventing GSAP interaction with the gamma-secretase substrate, APP-CTF. Thus, GSAP can serve as an amyloid-beta-lowering therapeutic target without affecting other key functions of gamma-secretase.
Figures

Comment in
-
Alzheimer's disease: Selectively tuning gamma-secretase.Nature. 2010 Sep 2;467(7311):36-7. doi: 10.1038/467036a. Nature. 2010. PMID: 20811445 No abstract available.
References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, Zhang L, Higgins GA, Parker EM. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

