Modeling core metabolism in cancer cells: surveying the topology underlying the Warburg effect
- PMID: 20811631
- PMCID: PMC2928278
- DOI: 10.1371/journal.pone.0012383
Modeling core metabolism in cancer cells: surveying the topology underlying the Warburg effect
Abstract
Background: Alterations on glucose consumption and biosynthetic activity of amino acids, lipids and nucleotides are metabolic changes for sustaining cell proliferation in cancer cells. Irrevocable evidence of this fact is the Warburg effect which establishes that cancer cells prefers glycolysis over oxidative phosphorylation to generate ATP. Regulatory action over metabolic enzymes has opened a new window for designing more effective anti-cancer treatments. This enterprise is not trivial and the development of computational models that contribute to identifying potential enzymes for breaking the robustness of cancer cells is a priority.
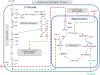
Methodology/principal findings: This work presents a constraint-base modeling of the most experimentally studied metabolic pathways supporting cancer cells: glycolysis, TCA cycle, pentose phosphate, glutaminolysis and oxidative phosphorylation. To evaluate its predictive capacities, a growth kinetics study for Hela cell lines was accomplished and qualitatively compared with in silico predictions. Furthermore, based on pure computational criteria, we concluded that a set of enzymes (such as lactate dehydrogenase and pyruvate dehydrogenase) perform a pivotal role in cancer cell growth, findings supported by an experimental counterpart.
Conclusions/significance: Alterations on metabolic activity are crucial to initiate and sustain cancer phenotype. In this work, we analyzed the phenotype capacities emerged from a constructed metabolic network conformed by the most experimentally studied pathways sustaining cancer cell growth. Remarkably, in silico model was able to resemble the physiological conditions in cancer cells and successfully identified some enzymes currently studied by its therapeutic effect. Overall, we supplied evidence that constraint-based modeling constitutes a promising computational platform to: 1) integrate high throughput technology and establish a crosstalk between experimental validation and in silico prediction in cancer cell phenotype; 2) explore the fundamental metabolic mechanism that confers robustness in cancer; and 3) suggest new metabolic targets for anticancer treatments. All these issues being central to explore cancer cell metabolism from a systems biology perspective.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

