Application of method suitability for drug permeability classification
- PMID: 20811966
- PMCID: PMC2976984
- DOI: 10.1208/s12248-010-9227-8
Application of method suitability for drug permeability classification
Abstract
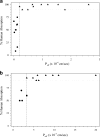
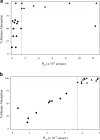
Experimental models of permeability in animals, excised tissues, cell monolayers, and artificial membranes are important during drug discovery and development as permeability is one of several factors affecting the intestinal absorption of oral drug products. The utility of these models is demonstrated by their ability to predict a drug's in vivo intestinal absorption. Within the various permeability models, there are differences in the performance of the assays, along with variability in animal species, tissue sources, and cell types, resulting in a variety of experimental permeability values for the same drug among laboratories. This has led to a need for assay standardization within laboratories to ensure applicability in the drug development process. Method suitability provides a generalized approach to standardize and validate a permeability model within a laboratory. First, assay methodology is optimized and validated for its various experimental parameters along with acceptance criteria for the assay. Second, the suitability of the model is demonstrated by a rank order relationship between experimental permeability values and human extent of absorption of known model compounds. Lastly, standard compounds are employed to classify a test drug's intestinal permeability and ensure assay reproducibility and quality. This review will provide examples of the different aspects method suitability for in situ (intestinal perfusions), ex vivo (everted intestinal sacs, diffusion chambers), and in vitro (cell monolayers, artificial membranes) experimental permeability models. Through assay standardization, reference standards, and acceptance criteria, method suitability assures the dependability of experimental data to predict a drug's intestinal permeability during discovery, development, and regulatory application.
Figures






Similar articles
-
The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests.Mol Pharm. 2006 Nov-Dec;3(6):686-94. doi: 10.1021/mp060042f. Mol Pharm. 2006. PMID: 17140256
-
Demonstrating suitability of the Caco-2 cell model for BCS-based biowaiver according to the recent FDA and ICH harmonised guidelines.J Pharm Pharmacol. 2019 Aug;71(8):1231-1242. doi: 10.1111/jphp.13111. Epub 2019 Jun 2. J Pharm Pharmacol. 2019. PMID: 31155721
-
Drug-permeability and transporter assays in Caco-2 and MDCK cell lines.Future Med Chem. 2011 Dec;3(16):2063-77. doi: 10.4155/fmc.11.149. Future Med Chem. 2011. PMID: 22098353 Review.
-
Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model.Eur J Med Chem. 2006 May;41(5):605-10. doi: 10.1016/j.ejmech.2006.01.013. Epub 2006 Mar 20. Eur J Med Chem. 2006. PMID: 16546303
-
Advances in cell-based permeability assays to screen drugs for intestinal absorption.Expert Opin Drug Discov. 2020 May;15(5):539-549. doi: 10.1080/17460441.2020.1735347. Epub 2020 Mar 10. Expert Opin Drug Discov. 2020. PMID: 32154737 Review.
Cited by
-
Pharmacokinetic Study of Withanosides and Withanolides from Withania somnifera Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS).Molecules. 2022 Feb 22;27(5):1476. doi: 10.3390/molecules27051476. Molecules. 2022. PMID: 35268576 Free PMC article.
-
Canine Intestinal Organoids as a Novel In Vitro Model of Intestinal Drug Permeability: A Proof-of-Concept Study.Cells. 2023 Apr 27;12(9):1269. doi: 10.3390/cells12091269. Cells. 2023. PMID: 37174669 Free PMC article.
-
A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids.Biology (Basel). 2025 Mar 6;14(3):270. doi: 10.3390/biology14030270. Biology (Basel). 2025. PMID: 40136526 Free PMC article.
-
Bioequivalence of oral products and the biopharmaceutics classification system: science, regulation, and public policy.Clin Pharmacol Ther. 2011 Sep;90(3):467-70. doi: 10.1038/clpt.2011.109. Epub 2011 Jul 20. Clin Pharmacol Ther. 2011. PMID: 21775984 Free PMC article. Review.
-
Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine.J Pharm Pharmacol. 2016 Aug;68(8):989-98. doi: 10.1111/jphp.12579. Epub 2016 Jun 10. J Pharm Pharmacol. 2016. PMID: 27283755 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

