Direct C-H arylation of electron-deficient heterocycles with arylboronic acids
- PMID: 20812741
- PMCID: PMC2946225
- DOI: 10.1021/ja1066459
Direct C-H arylation of electron-deficient heterocycles with arylboronic acids
Abstract
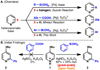
A direct arylation of a variety of electron-deficient heterocycles with arylboronic acids has been developed. This new reaction proceeds readily at room temperature using inexpensive reagents: catalytic silver(I) nitrate in the presence of persulfate co-oxidant. The scope with respect to heterocycle and boronic acid coupling partner is broad, and sensitive functional groups are tolerated. This method allows for rapid access to a variety of arylated heterocycles that would be more difficult to access with traditional methods.
Figures


References
-
- Suzuki A, Brown HC. Organic Synthesis Via Boranes. Volume 3. Milwaukee, WI: Aldrich; 2003.
-
-
For selected reviews on the Minisci reaction, see: Minisci F, Vismara E, Fontana F. Heterocycles. 1989;28:489. Minisci F, Fontana F, Vismara E. J. Heterocycl. Chem. 1990;27:79. Harrowven DC, Sutton BJ. Prog. Heterocycl. Chem. 2004;16:27.
-
-
- Minisci F, Vismara V, Fontana F, Morini G, Serravalle M, Giordano C. J. Org. Chem. 1986;51:4411.
-
-
For general reviews, see: Boulton AJ, McKillop A. In: Comprehensive Heterocyclic Chemistry. Katrizky AR, Rees CW, editors. Vol. 2. New York: Pergamon; 1984. pp. 262–270. Joule JA, Mills K. Heterocyclic Chemistry, Fourth Edition. Malden, MA: Blackwell; 2000. For a recent example with potassium t-Butoxide, suggested to be proceeding through a radical mechanism, see: Yanagisawa S, Ueda K, Tanaguchi T, Itami K. Org. Lett. 2008;10:4673.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

