Structure and function of the human breast cancer resistance protein (BCRP/ABCG2)
- PMID: 20812902
- PMCID: PMC2950214
- DOI: 10.2174/138920010792927325
Structure and function of the human breast cancer resistance protein (BCRP/ABCG2)
Abstract
The human breast cancer resistance protein (BCRP/ABCG2) is the second member of the G subfamily of the large ATP-binding cassette (ABC) transporter superfamily. BCRP was initially discovered in multidrug resistant breast cancer cell lines where it confers resistance to chemotherapeutic agents such as mitoxantrone, topotecan and methotrexate by extruding these compounds out of the cell. BCRP is capable of transporting non-chemotherapy drugs and xenobiotiocs as well, including nitrofurantoin, prazosin, glyburide, and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. BCRP is frequently detected at high levels in stem cells, likely providing xenobiotic protection. BCRP is also highly expressed in normal human tissues including the small intestine, liver, brain endothelium, and placenta. Therefore, BCRP has been increasingly recognized for its important role in the absorption, elimination, and tissue distribution of drugs and xenobiotics. At present, little is known about the transport mechanism of BCRP, particularly how it recognizes and transports a large number of structurally and chemically unrelated drugs and xenobiotics. Here, we review current knowledge of structure and function of this medically important ABC efflux drug transporter.
Conflict of interest statement
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Figures
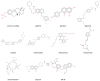
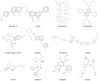

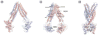
References
-
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59(1):8–13. - PubMed
-
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–5339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

