Chondroitin sulfate N-acetylgalactosaminyltransferase-1 is required for normal cartilage development
- PMID: 20812917
- PMCID: PMC2995422
- DOI: 10.1042/BJ20100847
Chondroitin sulfate N-acetylgalactosaminyltransferase-1 is required for normal cartilage development
Abstract
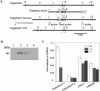
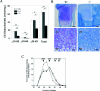
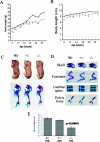
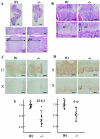
CS (chondroitin sulfate) is a glycosaminoglycan species that is widely distributed in the extracellular matrix. To understand the physiological roles of enzymes involved in CS synthesis, we produced CSGalNAcT1 (CS N-acetylgalactosaminyltransferase 1)-null mice. CS production was reduced by approximately half in CSGalNAcT1-null mice, and the amount of short-chain CS was also reduced. Moreover, the cartilage of the null mice was significantly smaller than that of wild-type mice. Additionally, type-II collagen fibres in developing cartilage were abnormally aggregated and disarranged in the homozygous mutant mice. These results suggest that CSGalNAcT1 is required for normal CS production in developing cartilage.
Figures
Similar articles
-
Chondroitin sulfate N-acetylgalactosaminyltransferase 1 is necessary for normal endochondral ossification and aggrecan metabolism.J Biol Chem. 2011 Feb 18;286(7):5803-12. doi: 10.1074/jbc.M110.159244. Epub 2010 Dec 10. J Biol Chem. 2011. PMID: 21148564 Free PMC article.
-
GlcUAβ1-3Galβ1-3Galβ1-4Xyl(2-O-phosphate) is the preferred substrate for chondroitin N-acetylgalactosaminyltransferase-1.J Biol Chem. 2015 Feb 27;290(9):5438-48. doi: 10.1074/jbc.M114.603266. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568321 Free PMC article.
-
Chondroitin sulfate N-acetylgalactosyltransferase-1 knockout shows milder phenotype in experimental autoimmune encephalomyelitis than in wild type.Glycobiology. 2021 Apr 1;31(3):260-265. doi: 10.1093/glycob/cwaa072. Glycobiology. 2021. PMID: 32839819
-
Roles of CSGalNAcT1, a key enzyme in regulation of CS synthesis, in neuronal regeneration and plasticity.Neurochem Int. 2018 Oct;119:77-83. doi: 10.1016/j.neuint.2017.10.001. Epub 2017 Oct 5. Neurochem Int. 2018. PMID: 28987564 Review.
-
The roles of chondroitin-4-sulfotransferase-1 in development and disease.Prog Mol Biol Transl Sci. 2010;93:113-32. doi: 10.1016/S1877-1173(10)93006-8. Prog Mol Biol Transl Sci. 2010. PMID: 20807643 Review.
Cited by
-
Reconsideration of the Semaphorin-3A Binding Motif Found in Chondroitin Sulfate Using Galnac4s-6st-Knockout Mice.Biomolecules. 2020 Oct 30;10(11):1499. doi: 10.3390/biom10111499. Biomolecules. 2020. PMID: 33143303 Free PMC article.
-
Human genetic disorders and knockout mice deficient in glycosaminoglycan.Biomed Res Int. 2014;2014:495764. doi: 10.1155/2014/495764. Epub 2014 Jul 13. Biomed Res Int. 2014. PMID: 25126564 Free PMC article. Review.
-
Chondroitin sulfate-E mediates estrogen-induced osteoanabolism.Sci Rep. 2015 Mar 11;5:8994. doi: 10.1038/srep08994. Sci Rep. 2015. PMID: 25759206 Free PMC article.
-
The different roles of aggrecan interaction domains.J Histochem Cytochem. 2012 Dec;60(12):987-96. doi: 10.1369/0022155412464376. Epub 2012 Sep 26. J Histochem Cytochem. 2012. PMID: 23019016 Free PMC article. Review.
-
Comparison of the effects of exercise with chondroitin sulfate on knee osteoarthritis in rabbits.J Orthop Surg Res. 2018 Jan 22;13(1):16. doi: 10.1186/s13018-018-0722-4. J Orthop Surg Res. 2018. PMID: 29357891 Free PMC article.
References
-
- Gandhi N. S., Mancera R. L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008;72:455–492. - PubMed
-
- Kitagawa H., Uyama T., Sugahara K. Molecular cloning and expression of a human chondroitin synthase. J. Biol. Chem. 2001;276:38721–38726. - PubMed
-
- Kitagawa H., Izumikawa T., Uyama T., Sugahara K. Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J. Biol. Chem. 2003;278:23666–23671. - PubMed
-
- Uyama T., Kitagawa H., Tamura J., Sugahara K. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: the key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 2002;277:8841–8846. - PubMed
-
- Uyama T., Kitagawa H., Tanaka J., Tamura J., Ogawa T., Sugahara K. Molecular cloning and expression of a second chondroitin N-acetylgalactosaminyltransferase involved in the initiation and elongation of chondroitin/dermatan sulfate. J. Biol. Chem. 2003;278:3072–3078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

