Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma
- PMID: 20813259
- PMCID: PMC2943345
- DOI: 10.1016/j.cell.2010.07.044
Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma
Abstract
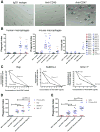
Monoclonal antibodies are standard therapeutics for several cancers including the anti-CD20 antibody rituximab for B cell non-Hodgkin lymphoma (NHL). Rituximab and other antibodies are not curative and must be combined with cytotoxic chemotherapy for clinical benefit. Here we report the eradication of human NHL solely with a monoclonal antibody therapy combining rituximab with a blocking anti-CD47 antibody. We identified increased expression of CD47 on human NHL cells and determined that higher CD47 expression independently predicted adverse clinical outcomes in multiple NHL subtypes. Blocking anti-CD47 antibodies preferentially enabled phagocytosis of NHL cells and synergized with rituximab. Treatment of human NHL-engrafted mice with anti-CD47 antibody reduced lymphoma burden and improved survival, while combination treatment with rituximab led to elimination of lymphoma and cure. These antibodies synergized through a mechanism combining Fc receptor (FcR)-dependent and FcR-independent stimulation of phagocytosis that might be applicable to many other cancers.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures






References
-
- A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. - PubMed
-
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
-
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. - PubMed
-
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

