New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention
- PMID: 20813271
- PMCID: PMC2933426
- DOI: 10.1016/j.clp.2010.05.006
New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention
Abstract
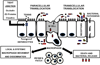
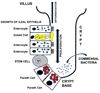
Bacterial translocation from the gastrointestinal tract is an important pathway initiating late-onset sepsis and necrotizing enterocolitis in very low-birth-weight infants. The emerging intestinal microbiota, nascent intestinal epithelia, naive immunity, and suboptimal nutrition (lack of breast milk) have roles in facilitating bacterial translocation. Feeding lactoferrin, probiotics, or prebiotics has presented exciting possibilities to prevent bacterial translocation in preterm infants, and clinical trials will identify the most safe and efficacious prevention and treatment strategies.
(c) 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–291. - PubMed
-
- Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. - PubMed
-
- Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill -- evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25(7):741–757. - PubMed
-
- Hunter CJ, Upperman JS, Ford HR, et al. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63(2):117–123. - PubMed

