Requiring an amyloid-beta1-42 biomarker for prodromal Alzheimer's disease or mild cognitive impairment does not lead to more efficient clinical trials
- PMID: 20813339
- PMCID: PMC2947209
- DOI: 10.1016/j.jalz.2010.07.004
Requiring an amyloid-beta1-42 biomarker for prodromal Alzheimer's disease or mild cognitive impairment does not lead to more efficient clinical trials
Abstract
Background: Low cerebrospinal fluid (CSF) amyloid-beta(1-42) concentration and high total-tau/Abeta(1-42) ratio have been recommended to support the diagnosis of prodromal Alzheimer's disease (AD) in patients with amnestic mild cognitive impairment (aMCI) and also to select patients for clinical trials (Shaw et al, Ann Neurol 2009;65:403-13; Dubois et al, Lancet Neurol 2007;6:734-46).
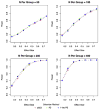
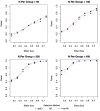
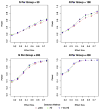
Methods: We tested this recommendation with clinical trials simulations using patients from the Alzheimer Disease Neuroimaging Initiative who fulfilled the following entry criteria: (1) aMCI, (2) aMCI with CSF Abeta(1-42) <or=192 mg/mL, (3) and aMCI with total-tau/Abeta(1-42) >0.39. For each criterion, we randomly resampled the database obtaining samples for 1000 trials for each trial scenario, planning for 1 or 2 year trials with samples from 50 to 400 patients per treatment or placebo group, with up to 40% dropouts, outcomes after using the AD assessment scale-cognitive subscale and clinical dementia rating scale with effect sizes ranging from 0.15 to 0.75, and calculated statistical power.
Findings: Approximately 70% to 74% of aMCI patients with CSF measures met biomarker criteria. The addition of the low Abeta(1-42) or high tau/Abeta(1-42) requirement resulted in minimal or no increase in the power of the trials compared with enrolling aMCI without requiring the biomarker criteria. Slightly larger mean differences between the placebo and treatment groups fulfilling biomarker criteria were offset by increased outcome variability within the groups.
Interpretations: Although patients with aMCI or patients with prodromal AD meeting CSF biomarkers criteria were slightly more cognitively impaired and showed greater decline than patients with aMCI diagnosed without considering the biomarkers, the requirement of biomarker-positive patients would most likely not result in more efficient clinical trials, and trials would take longer because fewer patients would be available. A CSF Abeta(1-42) marker, however, could be useful as an explanatory variable or covariate when warranted by the action of a drug.
Trial registration: ClinicalTrials.gov NCT00106899.
Copyright 2010 The Alzheimer
Conflict of interest statement
Conflict of interest statement: [During the 36-month window period before submission] LSS reports being an editor on the Cochrane Collaboration Dementia and Cognitive Improvement Group, which oversees systematic reviews of drugs for cognitive impairment and dementia; receiving a grant from the Alzheimer’s Association for a registry for dementia and cognitive impairment trials; receiving grant or research support from Baxter, Elan Pharmaceuticals, Johnson & Johnson, Eli Lilly, Myriad, Novartis, and Pfizer; and having served as a consultant for or receiving consulting fees from Abbott Laboratories, AC Immune, Allergan, Allon, Alzheimer Drug Discovery Foundation, AstraZeneca, Bristol-Myers Squibb, Elan, Eli Lilly, Exonhit, Forest, GlaxoSmithKline, Ipsen, Johnson & Johnson, Lundbeck, Myriad, Medavante, Medivation, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, Servier, Toyama, and Transition Therapeutics.
REK declares that he has no conflict of interest. GRC reports having served on data and safety monitoring committees for AntiSense, Sanofi-Aventis, Bayhill, BioMS, Daichi-Sankyo, Eli Lilly, GlaxoSmithKline, Genmab, Medivation, Ono, PTC Therapeutics, Vivus, NHLBI, NINDS, and the NMSS; and having served as a consultant to or receiving consulting fees from Alexion, Accentia, Bayer, Barofold, Biogen-Idec, CibaVision, Enzo, Eisai, Genentech, Millenium, Novartis, Consortium of Multiple Sclerosis Centers, Peptimmune, Klein-Buendel Incorporated, Incyte, Somnus, Teva, Visioneering Technologies.
Figures

Comment in
-
Requiring an amyloid-β1-42 biomarker for prodromal Alzheimer's disease or mild cognitive impairment does not lead to more efficient clinical trials.Alzheimers Dement. 2011 Mar;7(2):245-6; author reply 247-9. doi: 10.1016/j.jalz.2010.12.013. Alzheimers Dement. 2011. PMID: 21414558 No abstract available.
References
-
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. - PubMed
-
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

