Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice
- PMID: 20813896
- PMCID: PMC3012550
- DOI: 10.1182/blood-2010-07-295949
Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice
Abstract
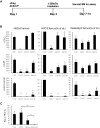
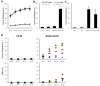
Inherited hematologic defects that lack an in vivo selective advantage following gene correction may benefit from effective yet minimally toxic cytoreduction of endogenous hematopoietic stem cells (HSCs) prior to transplantation of gene-modified HSCs. We studied the efficacy of administering a novel sequential treatment of parenteral ACK2, an antibody that blocks KIT, followed by low-dose irradiation (LD-IR) for conditioning of wild-type and X-linked chronic granulomatous disease (X-CGD) mice. In wild-type mice, combining ACK2 and LD-IR profoundly decreased endogenous competitive long-term HSC repopulating activity, and permitted efficient and durable donor-derived HSC engraftment after congenic transplantation. ACK2 alone was ineffective. The combination of ACK2 and LD-IR was also effective conditioning in X-CGD mice for engraftment of X-CGD donor HSCs transduced ex vivo with a lentiviral vector. We conclude that combining ACK2 with LD-IR is a promising approach to effectively deplete endogenous HSCs and facilitate engraftment of transplanted donor HSCs.
Figures
References
-
- Dinauer MC. Chronic granulomatous disease and other disorders of phagocyte function. Hematology Am Soc Hematol Educ Program. 2005:89–95. - PubMed
-
- Barese CN, Goebel WS, Dinauer MC. Gene therapy for chronic granulomatous disease. Expert Opin Biol Ther. 2004;4(9):1423–1434. - PubMed
-
- Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79(3):155–169. - PubMed
-
- Malech HL, Hickstein DD. Genetics, biology and clinical management of myeloid cell primary immune deficiencies: chronic granulomatous disease and leukocyte adhesion deficiency. Curr Opin Hematol. 2007;14(1):29–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

