The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria
- PMID: 20813918
- PMCID: PMC3116657
- DOI: 10.1126/science.1193637
The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria
Abstract
Ultraviolet UV-A and UV-B radiation is harmful to living systems, causing damage to biological macromolecules. An important strategy for dealing with UV exposure is the biosynthesis of small-molecule sunscreens. Among such metabolites, the mycosporine and mycosporine-like amino acids (MAAs) are remarkable for their wide phylogenetic distribution and their unique chemical structures. Here, we report the identification of a MAA biosynthetic gene cluster in a cyanobacterium and the discovery of analogous pathways in other sequenced organisms. We have expressed the cluster in a heterologous bacterial host and characterized all four biosynthetic enzymes in vitro. In addition to clarifying the origin of the MAAs, these efforts have revealed two unprecedented enzymatic strategies for imine formation.
Figures
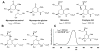


References
-
- Cockell CS, Knowland J. Biol Rev. 1999;74:311–345. - PubMed
-
- Bandaranayake WM. Nat Prod Rep. 1998;15:159–172. - PubMed
-
- Shick JM, Dunlap WC. Annu Rev Physiol. 2002;64:223–262. - PubMed
-
- Oren A, Gunde-Cimerman N. FEMS Microbiol Lett. 2007;269:1–10. - PubMed
-
- Dunlap WC, Shick JM. J Phycol. 1998;34:418–430.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

