Site-dependent E-cadherin cleavage and nuclear translocation in a metastatic colorectal cancer model
- PMID: 20813963
- PMCID: PMC2947300
- DOI: 10.2353/ajpath.2010.100079
Site-dependent E-cadherin cleavage and nuclear translocation in a metastatic colorectal cancer model
Abstract
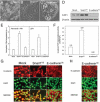
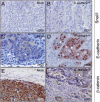
Metastases are frequently found during colorectal cancer diagnoses and are the main determinants of clinical outcome. The lack of reliable models of metastases has precluded their mechanistic understanding and our capacity to improve outcome. We studied the effect of E-cadherin and Snail1 expression on metastagenesis in a colorectal cancer model. We microinjected SW480-ADH human colorectal cancer cells, transfected with an empty vector (Mock) or overexpressing Snail1 (Snail1(OE)) or E-cadherin (E-cadherin(OE)), in the ceca of nude mice (eight per group) and analyzed tumor growth, dissemination, and Snail1, E-cadherin, β-catenin, and Presenilin1 (PS1) expression in local tumors and/or metastatic foci. Snail1(OE) cells disseminated only to lymph nodes, whereas Mock or E-cadherin(OE) cells spread to lymph nodes and peritoneums. Peritoneal tumor foci developed by E-cadherin(OE) cells presented an increase in E-cadherin proteolysis and nuclear translocation, and enhanced expression of proteolytically active PS1, which was linked to increased tumor growth and shortened mouse survival. Interestingly, local and lymph node tumors in mice bearing E-cadherin(OE) cells overexpressed E-cadherin, but they did not show E-cadherin proteolysis or nuclear translocation. Remarkably, E-cadherin nuclear translocation and enhanced expression of active PS1 were found in a patient with colorectal signet-ring cell carcinoma. In conclusion, we have established a colorectal cancer metastasis model in which E-cadherin proteolyis and nuclear translocation associates with aggressive foci growth only in the peritoneal microenvironment.
Figures







References
-
- DeVita VT, Jr, Lawrence TS, Rosenberg SA. Cancer: Principles & Practice of Oncology. Eighth edition. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 1144–1197.
-
- Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005;16:756–761. - PubMed
-
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. - PubMed
-
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

