Domain structure of virulence-associated response regulator PhoP of Mycobacterium tuberculosis: role of the linker region in regulator-promoter interaction(s)
- PMID: 20814030
- PMCID: PMC2966044
- DOI: 10.1074/jbc.M110.135822
Domain structure of virulence-associated response regulator PhoP of Mycobacterium tuberculosis: role of the linker region in regulator-promoter interaction(s)
Abstract
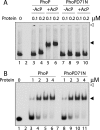
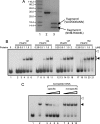
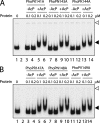
The PhoP and PhoR proteins from Mycobacterium tuberculosis form a highly specific two-component system that controls expression of genes involved in complex lipid biosynthesis and regulation of unknown virulence determinants. The several functions of PhoP are apportioned between a C-terminal effector domain (PhoPC) and an N-terminal receiver domain (PhoPN), phosphorylation of which regulates activation of the effector domain. Here we show that PhoPN, on its own, demonstrates PhoR-dependent phosphorylation. PhoPC, the truncated variant bearing the DNA binding domain, binds in vitro to the target site with affinity similar to that of the full-length protein. To complement the finding that residues spanning Met(1) to Arg(138) of PhoP constitute the minimal functional PhoPN, we identified Arg(150) as the first residue of the distal PhoPC domain capable of DNA binding on its own, thereby identifying an interdomain linker. However, coupling of two functional domains together in a single polypeptide chain is essential for phosphorylation-coupled DNA binding by PhoP. We discuss consequences of tethering of two domains on DNA binding and demonstrate that linker length and not individual residues of the newly identified linker plays a critical role in regulating interdomain interactions. Together, these results have implications for the molecular mechanism of transmission of conformation change associated with phosphorylation of PhoP that results in the altered DNA recognition by the C-terminal domain.
Figures




Similar articles
-
Structure of the DNA-binding domain of the response regulator PhoP from Mycobacterium tuberculosis.Biochemistry. 2007 Dec 25;46(51):14751-61. doi: 10.1021/bi700970a. Epub 2007 Dec 1. Biochemistry. 2007. PMID: 18052041 Free PMC article.
-
Unique N-terminal arm of Mycobacterium tuberculosis PhoP protein plays an unusual role in its regulatory function.J Biol Chem. 2013 Oct 4;288(40):29182-92. doi: 10.1074/jbc.M113.499905. Epub 2013 Aug 20. J Biol Chem. 2013. PMID: 23963455 Free PMC article.
-
A single-amino-acid substitution in the C terminus of PhoP determines DNA-binding specificity of the virulence-associated response regulator from Mycobacterium tuberculosis.J Mol Biol. 2010 May 21;398(5):647-56. doi: 10.1016/j.jmb.2010.03.056. Epub 2010 Apr 2. J Mol Biol. 2010. PMID: 20363229
-
[Mycobacterium tuberculosis PhoP system].Wei Sheng Wu Xue Bao. 2017 Apr 4;57(4):461-7. Wei Sheng Wu Xue Bao. 2017. PMID: 29756729 Review. Chinese.
-
PhoP, a key player in Mycobacterium tuberculosis virulence.Trends Microbiol. 2008 Nov;16(11):528-34. doi: 10.1016/j.tim.2008.08.006. Epub 2008 Oct 3. Trends Microbiol. 2008. PMID: 18835713 Review.
Cited by
-
Mycobacterium tuberculosis PhoP integrates stress response to intracellular survival by regulating cAMP level.Elife. 2024 May 13;13:RP92136. doi: 10.7554/eLife.92136. Elife. 2024. PMID: 38739431 Free PMC article.
-
Convergence of two global regulators to coordinate expression of essential virulence determinants of Mycobacterium tuberculosis.Elife. 2022 Nov 9;11:e80965. doi: 10.7554/eLife.80965. Elife. 2022. PMID: 36350294 Free PMC article.
-
The pathogenic mechanism of Mycobacterium tuberculosis: implication for new drug development.Mol Biomed. 2022 Dec 22;3(1):48. doi: 10.1186/s43556-022-00106-y. Mol Biomed. 2022. PMID: 36547804 Free PMC article. Review.
-
Structure of the response regulator PhoP from Mycobacterium tuberculosis reveals a dimer through the receiver domain.Biochemistry. 2011 Jul 5;50(26):5948-57. doi: 10.1021/bi2005575. Epub 2011 Jun 13. Biochemistry. 2011. PMID: 21634789 Free PMC article.
-
Solution structure of the PhoP DNA-binding domain from Mycobacterium tuberculosis.J Biomol NMR. 2015 Sep;63(1):111-7. doi: 10.1007/s10858-015-9965-0. Epub 2015 Jul 25. J Biomol NMR. 2015. PMID: 26209027 Free PMC article.
References
-
- Fontan P. A., Walters S., Smith I. (2004) Curr. Sci. 86, 122–134
-
- Av-Gay Y., Deretic V. (2005) in Tuberculosis and the Tubercle Bacillus (Cole S. T. ed) pp. 359–367, American Society for Microbiology Press, Washington, D. C.
-
- Pérez E., Samper S., Bordas Y., Guilhot C., Gicquel B., Martín C. (2001) Mol. Microbiol. 41, 179–187 - PubMed
-
- Walters S. B., Dubnau E., Kolesnikova I., Laval F., Daffe M., Smith I. (2006) Mol. Microbiol. 60, 312–330 - PubMed
-
- Gonzalo Asensio J., Maia C., Ferrer N. L., Barilone N., Laval F., Soto C. Y., Winter N., Daffé M., Gicquel B., Martín C., Jackson M. (2006) J. Biol. Chem. 281, 1313–1316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

