A phase 1/2, dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis
- PMID: 20814896
- PMCID: PMC3034044
- DOI: 10.1002/hep.23879
A phase 1/2, dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis
Abstract
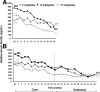
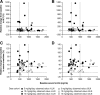
Hereditary hemochromatosis (HH) is characterized by increased intestinal iron absorption that may result in iron overload. Although phlebotomy is widely practiced, it is poorly tolerated or contraindicated in patients with anemias, severe heart disease, or poor venous access, and compliance can vary. The once-daily, oral iron chelator, deferasirox (Exjade) may provide an alternative treatment option. Patients with HH carrying the HFE gene who were homozygous for the Cys282Tyr mutation, serum ferritin levels of 300-2000 ng/mL, transferrin saturation ≥ 45%, and no known history of cirrhosis were enrolled in this dose-escalation study to characterize the safety and efficacy of deferasirox, comprising a core and an extension phase (each 24 weeks). Forty-nine patients were enrolled and received starting deferasirox doses of 5 (n = 11), 10 (n = 15), or 15 (n = 23) mg/kg/day. Adverse events were generally dose-dependent, the most common being diarrhea, headache, and nausea (n = 18, n = 10, and n = 8 in the core and n = 1, n = 1, and n = 0 in the extension, respectively). More patients in the 15 mg/kg/day than in the 5 or 10 mg/kg/day cohorts experienced increases in alanine aminotransferase and serum creatinine levels during the 48-week treatment period; six patients had alanine aminotransferase > 3 × baseline and greater than the upper limit of normal range, and eight patients had serum creatinine > 33% above baseline and greater than upper limit of normal on two consecutive occasions. After receiving deferasirox for 48 weeks, median serum ferritin levels decreased by 63.5%, 74.8%, and 74.1% in the 5, 10, and 15 mg/kg/day cohorts, respectively. In all cohorts, median serum ferritin decreased to < 250 ng/mL.
Conclusion: Deferasirox doses of 5, 10, and 15 mg/kg/day can reduce iron burden in patients with HH. Based on the safety and efficacy results, starting deferasirox at 10 mg/kg/day appears to be most appropriate for further study in this patient population.
Figures


References
-
- Morrison ED, Brandhagen DJ, Phatak PD, Barton JC, Krawitt EL, El-Serag HB, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med. 2003;138:627–633. - PubMed
-
- Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. - PubMed
-
- Bomford A. Genetics of haemochromatosis. Lancet. 2002;360:1673–1681. - PubMed
-
- Pietrangelo A. Iron chelation beyond transfusion iron overload. Am J Hematol. 2007;82:1142–1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
