Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm
- PMID: 20815087
- PMCID: PMC2950924
- DOI: 10.1002/pmic.201000002
Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm
Abstract
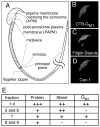
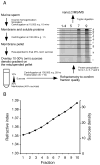
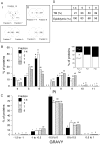
Mammalian sperm are transcriptionally and translationally inactive. To meet changing needs in the epididymis and female tract, they rely heavily on post-translational modifications and protein acquisition/degradation. Membrane rafts are sterol and sphingolipid-enriched micro-domains that organize and regulate various pathways. Rafts have significance in sperm by transducing the stimulus of sterol efflux into changes in intracellular signaling that confer fertilization competence. We recently characterized three biochemically distinct sub-types of sperm rafts, and now present profiles for proteins targeting to and associating with these sub-types, along with a fraction largely comprised of "non-raft" domains. Proteomics analysis using a gel-based LC-MS/MS approach identified 190 strictly validated proteins in the raft sub-types. Interestingly, many of these are known to be expressed in the epididymis, where sperm membrane composition matures. To investigate potential roles for rafts in epididymal protein acquisition, we compared the expression and localization of two different sterol-interacting proteins, apolipoprotein-A1 (apoA1) and prominin-1 (prom1) in sperm from different zones. We found that apoA1 was gradually added to the plasma membrane overlying the acrosome, whereas prom1 was not, suggesting different mechanisms for raft protein acquisition. Our results define raft-associating proteins, demonstrate functional similarities and differences among raft sub-types, and provide insights into raft-mediated epididymal protein acquisition.
Conflict of interest statement
Conflict of interest statement
No direct financial gain will be achieved by publication of our manuscript. No potential commercial partners have been involved in the study design, performance of work, analysis of data, preparation of the manuscript, or decision to submit.
Figures

Similar articles
-
Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts.J Cell Physiol. 2009 Mar;218(3):537-48. doi: 10.1002/jcp.21623. J Cell Physiol. 2009. PMID: 19006178 Free PMC article.
-
Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida.J Cell Physiol. 2012 Dec;227(12):3876-86. doi: 10.1002/jcp.24099. J Cell Physiol. 2012. PMID: 22552861
-
Na/K-ATPase regulates bovine sperm capacitation through raft- and non-raft-mediated signaling mechanisms.Mol Reprod Dev. 2017 Nov;84(11):1168-1182. doi: 10.1002/mrd.22879. Epub 2017 Sep 27. Mol Reprod Dev. 2017. PMID: 28833817
-
Sperm head membrane reorganisation during capacitation.Int J Dev Biol. 2008;52(5-6):473-80. doi: 10.1387/ijdb.082583bg. Int J Dev Biol. 2008. PMID: 18649260 Review.
-
Lipid raft proteomics: more than just detergent-resistant membranes.Subcell Biochem. 2007;43:35-47. doi: 10.1007/978-1-4020-5943-8_4. Subcell Biochem. 2007. PMID: 17953390 Review.
Cited by
-
Association between Yili goose sperm motility and expression profiles of mRNA and miRNA in testis.BMC Genomics. 2023 Oct 24;24(1):640. doi: 10.1186/s12864-023-09727-1. BMC Genomics. 2023. PMID: 37875805 Free PMC article.
-
Drosophila sperm proteome evolution: Insights from comparative genomic approaches.Spermatogenesis. 2012 Jul 1;2(3):213-223. doi: 10.4161/spmg.21748. Spermatogenesis. 2012. PMID: 23087838 Free PMC article.
-
Bottom-up approach to deciphering the targets of the ubiquitin-proteasome system in porcine sperm capacitation.Sci Rep. 2024 Aug 29;14(1):20159. doi: 10.1038/s41598-024-71056-4. Sci Rep. 2024. PMID: 39215164 Free PMC article.
-
Hydrogen Sulfide and/or Ammonia Reduces Spermatozoa Motility through AMPK/AKT Related Pathways.Sci Rep. 2016 Nov 24;6:37884. doi: 10.1038/srep37884. Sci Rep. 2016. PMID: 27883089 Free PMC article.
-
Organization of Membrane Rafts in Chicken Sperm.J Poult Sci. 2016 Jul 25;53(3):233-239. doi: 10.2141/jpsa.0150160. J Poult Sci. 2016. PMID: 32908389 Free PMC article.
References
-
- Cornwall GA, Vreeburg JT, Holland MK, Orgebin-Crist MC. Interactions of labeled epididymal secretory proteins with spermatozoa after injection of 35S-methionine in the mouse. Biol Reprod. 1990;43:121–129. - PubMed
-
- Frenette G, Girouard J, Sullivan R. Comparison Between Epididymosomes Collected in the Intraluminal Compartment of the Bovine Caput and Cauda Epididymidis. Biol Reprod. 2006;75:885–890. - PubMed
-
- Chang MC. Fertilizing Capacity of Spermatozoa deposited into the Fallopian Tubes. Nature. 1951;168:697–698. - PubMed
-
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. - PubMed
-
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

