Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus
- PMID: 20815704
- PMCID: PMC2941567
- DOI: 10.1086/656365
Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus
Abstract
Background: Highly pathogenic avian influenza viruses of the H5N1 subtype continue to cross the species barrier to infect humans and cause severe disease. It has been suggested that an exaggerated immune response contributes to the pathogenesis of H5N1 virus infection in mammals. In particular, H5N1 virus infections are associated with a high expression of the proinflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α).
Methods: We investigated the compounding affects of both cytokines on the outcome of H5N1 virus disease by using triple mutant mice deficient in 3 signaling receptors, TNF-R1, TNF-R2, and IL-1-RI.
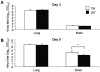
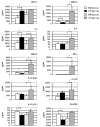
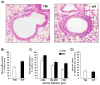
Results: Triple mutant mice exhibited reduced morbidity and a significant delay in mortality following lethal challenge with a lethal H5N1 virus, whereas no such differences were observed with the less virulent A/PR/8/34 (H1N1) virus. H5N1-infected triple mutant mice displayed diminished cytokine production in lung tissue and a quantifiable decrease of macrophages and neutrophils in the lungs postinfection. Moreover, morphometric analysis of airway sections revealed less extensive inflammation in H5N1-infected triple mutant mice, compared with infected wild-type mice.
Conclusions: The combined signaling from the TNF or IL-1 receptors promotes maximal lung inflammation that may contribute to the severity of disease caused by H5N1 virus infection.
Figures


Similar articles
-
Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice.J Virol. 2007 Mar;81(6):2736-44. doi: 10.1128/JVI.02336-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182684 Free PMC article.
-
CLEC5A-Mediated Enhancement of the Inflammatory Response in Myeloid Cells Contributes to Influenza Virus Pathogenicity In Vivo.J Virol. 2016 Dec 16;91(1):e01813-16. doi: 10.1128/JVI.01813-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795434 Free PMC article.
-
Highly pathogenic avian influenza A H5N1 and pandemic H1N1 virus infections have different phenotypes in Toll-like receptor 3 knockout mice.J Gen Virol. 2014 Sep;95(Pt 9):1870-1879. doi: 10.1099/vir.0.066258-0. Epub 2014 May 30. J Gen Virol. 2014. PMID: 24878639 Free PMC article.
-
[Cytokine storm in avian influenza].Mikrobiyol Bul. 2008 Apr;42(2):365-80. Mikrobiyol Bul. 2008. PMID: 18697437 Review. Turkish.
-
Into the eye of the cytokine storm.Microbiol Mol Biol Rev. 2012 Mar;76(1):16-32. doi: 10.1128/MMBR.05015-11. Microbiol Mol Biol Rev. 2012. PMID: 22390970 Free PMC article. Review.
Cited by
-
IL-36α exerts pro-inflammatory effects in the lungs of mice.PLoS One. 2012;7(9):e45784. doi: 10.1371/journal.pone.0045784. Epub 2012 Sep 20. PLoS One. 2012. PMID: 23029241 Free PMC article.
-
Genome-wide profiling of microRNAs reveals novel insights into the interactions between H9N2 avian influenza virus and avian dendritic cells.Oncogene. 2018 Aug;37(33):4562-4580. doi: 10.1038/s41388-018-0279-z. Epub 2018 May 10. Oncogene. 2018. PMID: 29743596
-
Influenza A Viruses Replicate Productively in Mouse Mastocytoma Cells (P815) and Trigger Pro-inflammatory Cytokine and Chemokine Production through TLR3 Signaling Pathway.Front Microbiol. 2017 Jan 12;7:2130. doi: 10.3389/fmicb.2016.02130. eCollection 2016. Front Microbiol. 2017. PMID: 28127293 Free PMC article.
-
Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung.Mucosal Immunol. 2012 May;5(3):258-66. doi: 10.1038/mi.2012.2. Epub 2012 Feb 1. Mucosal Immunol. 2012. PMID: 22294047 Free PMC article.
-
Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection.Virulence. 2013 Aug 15;4(6):537-42. doi: 10.4161/viru.25779. Epub 2013 Jul 17. Virulence. 2013. PMID: 23863601 Free PMC article. Review.
References
-
- World Health Organization. http://www.who.int/csr/disease/avian_influenza/country/en/index.html.
-
- Tran TH, Nguyen TL, Nguyen TD, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. - PubMed
-
- Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. - PubMed
-
- Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–75. - PubMed
-
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

