Overcoming platinum resistance in ovarian carcinoma
- PMID: 20815774
- PMCID: PMC2962713
- DOI: 10.1517/13543784.2010.515585
Overcoming platinum resistance in ovarian carcinoma
Abstract
Importance of the field: Ovarian cancer remains a deadly malignancy because most patients develop recurrent disease that is resistant to chemotherapy, including platinum. Because response rates for current treatment regimens are relatively similar and unfortunately low, no standard chemotherapy for platinum-resistant ovarian cancer exists.
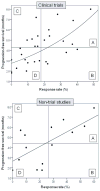
Areas covered in this review: A systematic literature review of clinical studies published between January 2005 and March 2010 was conducted using search engines, PubMed and MEDLINE with the entry keywords 'ovarian cancer' and 'platinum resistance'. This search revealed 40 clinical trials (1793 patients).
What the reader will gain: Gemcitabine was the most common drug used in clinical trials reporting higher response rates, ≥ +1 SD of overall response rate (5 out of 8). Gemcitabine-based combination therapy showed an average response rate of 27.2% (95% CI, 22.4-32.0). Combination of gemcitabine and pegylated liposomal doxorubicin (PLD) was the most common regimen (n = 3) and was associated with possible additive effects in platinum-resistant ovarian cancer patients: response rate, gemcitabine alone 6.1%, PLD alone 19.8%, and gemcitabine with PLD 28.7% (95% CI, 20.4-37.0), respectively.
Take home message: Analysis of recent clinical trials showed that gemcitabine-based combination chemotherapy was associated with the highest antitumor effects in platinum-resistant ovarian cancer patients during the study period.
Conflict of interest statement
K Matsuo is supported by the GCF/OCRF Ann Schreiber Ovarian Cancer Research grant, the Betty Anne Asche Murray Distinguished Professorship and an award from the Meyer and Ida Gordon Foundation #2. Portions of this work were supported by the NIH (P50 CA083639, P50 CA098258, CA128797, RC2GM092599), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC073399, OC093146, BC085265) to AK Sood and K Matsuo. YG Lin is supported by the Woman’s Cancer Programme, University of Southern California. L Roman has nothing to disclose.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Omura G, Blessing JA, Ehrlich CE, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer. 1986;57:1725–30. - PubMed
-
- Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–7. - PubMed
-
- Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials