Mtmr8 is essential for vasculature development in zebrafish embryos
- PMID: 20815916
- PMCID: PMC2944161
- DOI: 10.1186/1471-213X-10-96
Mtmr8 is essential for vasculature development in zebrafish embryos
Abstract
Background: Embryonic morphogenesis of vascular and muscular systems is tightly coordinated, and a functional cooperation of Mtmr8 with PI3K in actin filament modeling and muscle development has been revealed in zebrafish. Here, we attempt to explore the function of Mtmr8 in vasculature development parallel to its function in muscle development.
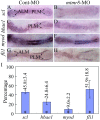
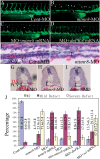
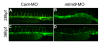
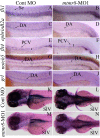
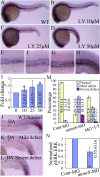
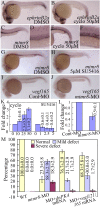
Results: During early stage of somitogenesis, mtmr8 expression was detected in both somitic mesodem and ventral mesoderm. Knockdown of mtmr8 by morpholino impairs arterial endothelial marker expression, and results in endothelial cell reduction and vasculogenesis defects, such as retardation in intersegmental vessel development and interruption of trunk dorsal aorta. Moreover, mtmr8 morphants show loss of arterial endothelial cell identity in dorsal aorta, which is effectively rescued by low concentration of PI3K inhibitor, and by over-expression of dnPKA mRNA or vegf mRNA. Interestingly, mtmr8 expression is up-regulated when zebrafish embryos are treated with specific inhibitor of Hedgehog pathway that abolishes arterial marker expression.
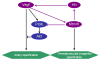
Conclusion: These data indicate that Mtmr8 is essential for vasculature development in zebrafish embryos, and may play a role in arterial specification through repressing PI3K activity. It is suggested that Mtmr8 should represent a novel element of the Hedgehog/PI3K/VEGF signaling cascade that controls arterial specification.
Figures

Similar articles
-
Cooperation of Mtmr8 with PI3K regulates actin filament modeling and muscle development in zebrafish.PLoS One. 2009;4(3):e4979. doi: 10.1371/journal.pone.0004979. Epub 2009 Mar 26. PLoS One. 2009. PMID: 19325702 Free PMC article.
-
Vegf signaling promotes vascular endothelial differentiation by modulating etv2 expression.Dev Biol. 2017 Apr 15;424(2):147-161. doi: 10.1016/j.ydbio.2017.03.005. Epub 2017 Mar 7. Dev Biol. 2017. PMID: 28279709 Free PMC article.
-
Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate.Dev Biol. 2010 May 1;341(1):196-204. doi: 10.1016/j.ydbio.2010.02.028. Epub 2010 Feb 26. Dev Biol. 2010. PMID: 20193674 Free PMC article.
-
Zebrafish genetics and formation of embryonic vasculature.Curr Top Dev Biol. 2005;71:53-81. doi: 10.1016/S0070-2153(05)71002-4. Curr Top Dev Biol. 2005. PMID: 16344102 Review.
-
Vascular endothelial growth factor and its receptors in embryonic zebrafish blood vessel development.Curr Top Dev Biol. 2004;62:127-52. doi: 10.1016/S0070-2153(04)62005-9. Curr Top Dev Biol. 2004. PMID: 15522741 Review.
Cited by
-
A conserved myotubularin-related phosphatase regulates autophagy by maintaining autophagic flux.J Cell Biol. 2020 Nov 2;219(11):e201909073. doi: 10.1083/jcb.201909073. J Cell Biol. 2020. PMID: 32915229 Free PMC article.
-
Phosphoinositides: tiny lipids with giant impact on cell regulation.Physiol Rev. 2013 Jul;93(3):1019-137. doi: 10.1152/physrev.00028.2012. Physiol Rev. 2013. PMID: 23899561 Free PMC article. Review.
-
Detection of genomic structural variations in Guizhou indigenous pigs and the comparison with other breeds.PLoS One. 2018 Mar 20;13(3):e0194282. doi: 10.1371/journal.pone.0194282. eCollection 2018. PLoS One. 2018. PMID: 29558483 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

