Evidence of D-phenylglycine as delivering tool for improving L-dopa absorption
- PMID: 20815935
- PMCID: PMC2941486
- DOI: 10.1186/1423-0127-17-71
Evidence of D-phenylglycine as delivering tool for improving L-dopa absorption
Abstract
Background: L-dopa has been used for Parkinson's disease management for a long time. However, its wide variety in the rate and the extent of absorption remained challenge in designing suitable therapeutic regime. We report here a design of using D-phenylglycine to guard L-dopa for better absorption in the intestine via intestinal peptide transporter I (PepT1).
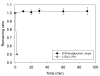
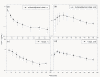
Methods: D-phenylglycine was chemically attached on L-dopa to form D-phenylglycine-L-dopa as a dipeptide prodrug of L-dopa. The cross-membrane transport of this dipeptide and L-dopa via PepT1 was compared in brush-boarder membrane vesicle (BBMV) prepared from rat intestine. The intestinal absorption was compared by in situ jejunal perfusion in rats. The pharmacokinetics after i.v. and p.o. administration of both compounds were also compared in Wistar rats. The striatal dopamine released after i.v. administration of D-phenylglycine-L-dopa was collected by brain microdialysis and monitored by HPLC. Anti-Parkinsonism effect was determined by counting the rotation of 6-OHDA-treated unilateral striatal lesioned rats elicited rotation with (+)-methamphetamine (MA).
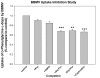
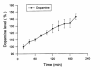
Results: The BBMV uptake of D-phenylglycine-L-dopa was inhibited by Gly-Pro, Gly-Phe and cephradine, the typical PepT1 substrates, but not by amino acids Phe or L-dopa. The cross-membrane permeability (Pm*) determined in rat jejunal perfusion of D-phenylglycine-L-dopa was higher than that of L-dopa (2.58 ± 0.14 vs. 0.94 ± 0.10). The oral bioavailability of D-phenylglycine-L-dopa was 31.7 times higher than that of L-dopa in rats. A sustained releasing profile of striatal dopamine was demonstrated after i. v. injection of D-phenylglycine-L-dopa (50 mg/kg), indicated that D-phenylglycine-L-dopa might be a prodrug of dopamine. D-phenylglycine-L-dopa was more efficient than L-dopa in lowering the rotation of unilateral striatal lesioned rats (19.1 ± 1.7% vs. 9.9 ± 1.4%).
Conclusion: The BBMV uptake studies indicated that D-phenylglycine facilitated the transport of L-dopa through the intestinal PepT1 transporter. The higher jejunal permeability and the improved systemic bioavailability of D-phenylglycine-L-dopa in comparison to that of l-dopa suggested that D-phenylglycine is an effective delivery tool for improving the oral absorption of drugs like L-dopa with unsatisfactory pharmacokinetics. The gradual release of dopamine in brain striatum rendered this dipeptide as a potential dopamine sustained-releasing prodrug.
Figures
Similar articles
-
Intestinal absorption studies on peptide mimetic alpha-methyldopa prodrugs.J Pharm Pharmacol. 1996 Mar;48(3):270-6. doi: 10.1111/j.2042-7158.1996.tb05915.x. J Pharm Pharmacol. 1996. PMID: 8737052
-
Unpredictable rotational responses to L-dopa in the rat model of Parkinson's disease: the role of L-dopa pharmacokinetics and striatal dopamine depletion.Basic Clin Pharmacol Toxicol. 2012 Feb;110(2):162-70. doi: 10.1111/j.1742-7843.2011.00782.x. Epub 2011 Sep 13. Basic Clin Pharmacol Toxicol. 2012. PMID: 21848668
-
Improvement of L-dopa absorption by dipeptidyl derivation, utilizing peptide transporter PepT1.J Pharm Sci. 1998 Dec;87(12):1542-6. doi: 10.1021/js980186o. J Pharm Sci. 1998. PMID: 10189264
-
Peculiarities of L: -DOPA treatment of Parkinson's disease.Amino Acids. 2005 Mar;28(2):157-64. doi: 10.1007/s00726-005-0162-4. Epub 2005 Mar 9. Amino Acids. 2005. PMID: 15750845 Review.
-
Dopamine and Levodopa Prodrugs for the Treatment of Parkinson's Disease.Molecules. 2017 Dec 25;23(1):40. doi: 10.3390/molecules23010040. Molecules. 2017. PMID: 29295587 Free PMC article. Review.
Cited by
-
Transporter-Mediated Drug Delivery.Molecules. 2023 Jan 24;28(3):1151. doi: 10.3390/molecules28031151. Molecules. 2023. PMID: 36770817 Free PMC article. Review.
-
Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease.Compr Physiol. 2018 Mar 25;8(2):731-760. doi: 10.1002/cphy.c170032. Compr Physiol. 2018. PMID: 29687900 Free PMC article. Review.
-
Molecular Variability in Levodopa Absorption and Clinical Implications for the Management of Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1353-1368. doi: 10.3233/JPD-240036. J Parkinsons Dis. 2024. PMID: 39240647 Free PMC article. Review.
-
Rapid and Sensitive Quantification of Intracellular Glycyl-Sarcosine for Semi-High-Throughput Screening for Inhibitors of PEPT-1.Pharmaceutics. 2021 Jul 3;13(7):1019. doi: 10.3390/pharmaceutics13071019. Pharmaceutics. 2021. PMID: 34371711 Free PMC article.
References
-
- Juncos JL. Levodopa: pharmacology, pharmacokinetics, and pharmacodynamics. Neurol Clin. 1992;10:487–509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

