Canonical and non-canonical Notch ligands
- PMID: 20816393
- PMCID: PMC4286395
- DOI: 10.1016/S0070-2153(10)92003-6
Canonical and non-canonical Notch ligands
Abstract
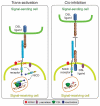
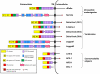
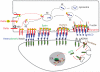
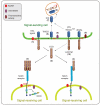
Notch signaling induced by canonical Notch ligands is critical for normal embryonic development and tissue homeostasis through the regulation of a variety of cell fate decisions and cellular processes. Activation of Notch signaling is normally tightly controlled by direct interactions with ligand-expressing cells, and dysregulated Notch signaling is associated with developmental abnormalities and cancer. While canonical Notch ligands are responsible for the majority of Notch signaling, a diverse group of structurally unrelated noncanonical ligands has also been identified that activate Notch and likely contribute to the pleiotropic effects of Notch signaling. Soluble forms of both canonical and noncanonical ligands have been isolated, some of which block Notch signaling and could serve as natural inhibitors of this pathway. Ligand activity can also be indirectly regulated by other signaling pathways at the level of ligand expression, serving to spatiotemporally compartmentalize Notch signaling activity and integrate Notch signaling into a molecular network that orchestrates developmental events. Here, we review the molecular mechanisms underlying the dual role of Notch ligands as activators and inhibitors of Notch signaling. Additionally, evidence that Notch ligands function independent of Notch is presented. We also discuss how ligand posttranslational modification, endocytosis, proteolysis, and spatiotemporal expression regulate their signaling activity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Aho S. Soluble form of Jagged1: unique product of epithelial keratinocytes and a regulator of keratinocyte differentiation. J Cell Biochem. 2004;92:1271–1281. - PubMed
-
- Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

