Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion
- PMID: 20816837
- PMCID: PMC2997213
- DOI: 10.1053/j.gastro.2010.08.047
Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion
Abstract
Background & aims: Hypochlorhydria during Helicobacter pylori infection inhibits gastric Sonic Hedgehog (Shh) expression. We investigated whether acid-secretory mechanisms regulate Shh gene expression through intracellular calcium (Ca2(+)(i))-dependent protein kinase C (PKC) or cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) activation.
Methods: We blocked Hedgehog signaling by transgenically overexpressing a secreted form of the Hedgehog interacting protein-1, a natural inhibitor of hedgehog ligands, which induced hypochlorhydria. Gadolinium, ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) + 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), PKC-overexpressing adenoviruses, and PKC inhibitors were used to modulate Ca(2+)(i)-release, PKC activity, and Shh gene expression in primary gastric cell, organ, and AGS cell line cultures. PKA hyperactivity was induced in the H(+)/K(+)-β-cholera-toxin-overexpressing mice.
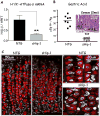
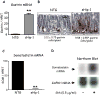
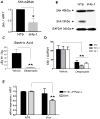
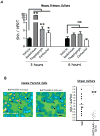
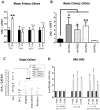
Results: Mice that expressed secreted hedgehog-interacting protein-1 had lower levels of gastric acid (hypochlorhydria), reduced production of somatostatin, and increased gastrin gene expression. Hypochlorhydria in these mice repressed Shh gene expression, similar to the levels obtained with omeprazole treatment of wild-type mice. However, Shh expression also was repressed in the hyperchlorhydric H(+)/K(+)-β-cholera-toxin model with increased cAMP, suggesting that the regulation of Shh was not solely acid-dependent, but pertained to specific acid-stimulatory signaling pathways. Based on previous reports that Ca(2+)(i) release also stimulates acid secretion in parietal cells, we showed that gadolinium-, thapsigargin-, and carbachol-mediated release of Ca(2+)(i) induced Shh expression. Ca(2+)-chelation with BAPTA + EGTA reduced Shh expression. Overexpression of PKC-α, -β, and -δ (but not PKC-ϵ) induced an Shh gene expression. In addition, phorbol esters induced a Shh-regulated reporter gene.
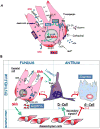
Conclusions: Secretagogues that stimulate gastric acid secretion induce Shh gene expression through increased Ca(2+)(i)-release and PKC activation. Shh might be the ligand transducing changes in gastric acidity to the regulation of G-cell secretion of gastrin.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Morphogens and the parietal cell: shaping up acid secretion.Gastroenterology. 2010 Dec;139(6):1830-3. doi: 10.1053/j.gastro.2010.10.035. Epub 2010 Oct 26. Gastroenterology. 2010. PMID: 21029799 No abstract available.
References
-
- van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. - PubMed
-
- El-Zaatari M, Grabowska AM, McKenzie AJ, Powe DG, Scotting PJ, Watson SA. Cyclopamine inhibition of the sonic hedgehog pathway in the stomach requires concomitant acid inhibition. Regul Pept. 2008;146:131–9. - PubMed
-
- Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–8. - PubMed
-
- Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, DLG, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

