Elucidating biological risk factors in suicide: role of protein kinase A
- PMID: 20817068
- PMCID: PMC3026860
- DOI: 10.1016/j.pnpbp.2010.08.025
Elucidating biological risk factors in suicide: role of protein kinase A
Abstract
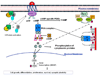
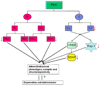
Suicide is a major public health concern. Although there have been several studies of suicidal behavior that focused on the roles of psychosocial and sociocultural factors, these factors are of too little predictive value to be clinically useful. Therefore, research on the biological perspective of suicide has gained a stronghold and appears to provide a promising approach to identify biological risk factors associated with suicidal behavior. Recent studies demonstrate that an alteration in synaptic and structural plasticity is key to affective illnesses and suicide. Signal transduction molecules play an important role in such plastic events. Protein kinase A (PKA) is a crucial enzyme in the adenylyl cyclase signal transduction pathway and is involved in regulating gene transcription, cell survival, and plasticity. In this review, we critically and comprehensively discuss the role of PKA in suicidal behavior. Because stress is an important component of suicide, we also discuss whether stress affects PKA and how this may be associated with suicidal behavior. In addition, we also discuss the functional significance of the findings regarding PKA by describing the role of important PKA substrates (i.e., Rap1, cyclic adenosine monophosphate response element binding protein, and target gene brain-derived neurotrophic factor). These studies suggest the interesting possibility that PKA and related signaling molecules may serve as important neurobiological factors in suicide and may be relevant in target-specific therapeutic interventions for these disorders.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Alteration of second messengers during acute cerebral ischemia - adenylate cyclase, cyclic AMP-dependent protein kinase, and cyclic AMP response element binding protein.Prog Neurobiol. 2001 Oct;65(2):173-207. doi: 10.1016/s0301-0082(01)00002-8. Prog Neurobiol. 2001. PMID: 11403878 Review.
-
Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway.J Biol Chem. 2000 Nov 3;275(44):34433-41. doi: 10.1074/jbc.M004728200. J Biol Chem. 2000. PMID: 10950954
-
Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor.J Cell Biol. 2003 Feb 17;160(4):487-93. doi: 10.1083/jcb.200209105. Epub 2003 Feb 10. J Cell Biol. 2003. PMID: 12578910 Free PMC article.
-
Signal transduction abnormalities in suicide: focus on phosphoinositide signaling system.CNS Neurol Disord Drug Targets. 2013 Nov;12(7):941-53. doi: 10.2174/18715273113129990097. CNS Neurol Disord Drug Targets. 2013. PMID: 24040801 Review.
-
Cyclic adenosine 3'-5'-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac).Mol Cell Endocrinol. 2014 Mar 5;383(1-2):193-202. doi: 10.1016/j.mce.2013.12.006. Epub 2013 Dec 25. Mol Cell Endocrinol. 2014. PMID: 24373949
Cited by
-
Regulating the ubiquitin/proteasome pathway via cAMP-signaling: neuroprotective potential.Cell Biochem Biophys. 2013 Sep;67(1):55-66. doi: 10.1007/s12013-013-9628-2. Cell Biochem Biophys. 2013. PMID: 23686612 Free PMC article. Review.
-
Enoxacin Elevates MicroRNA Levels in Rat Frontal Cortex and Prevents Learned Helplessness.Front Psychiatry. 2014 Feb 10;5:6. doi: 10.3389/fpsyt.2014.00006. eCollection 2014. Front Psychiatry. 2014. PMID: 24575053 Free PMC article.
-
DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation.J Psychiatr Res. 2017 Jun;89:115-124. doi: 10.1016/j.jpsychires.2017.02.005. Epub 2017 Feb 16. J Psychiatr Res. 2017. PMID: 28246044 Free PMC article.
-
MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects.PLoS One. 2012;7(3):e33201. doi: 10.1371/journal.pone.0033201. Epub 2012 Mar 9. PLoS One. 2012. PMID: 22427989 Free PMC article.
-
Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects.Neuropsychopharmacology. 2021 Apr;46(5):900-910. doi: 10.1038/s41386-020-00861-y. Epub 2020 Sep 12. Neuropsychopharmacology. 2021. PMID: 32919404 Free PMC article.
References
-
- Adams JA, McGlone ML, Gibson R, Taylor SS. Phosphorylation modulates catalytic function and regulation in the cAMP- dependent protein kinase. Biochemistry. 1995;34:2447–2454. - PubMed
-
- Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22:59–65. - PubMed
-
- Altamura AC, Bassetti R, Bignotti S, Pioli R, Mundo E. Clinical variables related to suicide attempts in schizophrenic patients: a retrospective study. Schizophr Res. 2003;60:47–55. - PubMed
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsey RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

