Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity
- PMID: 20817616
- PMCID: PMC2951474
- DOI: 10.1016/j.devcel.2010.08.004
Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity
Abstract
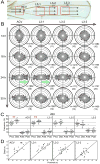
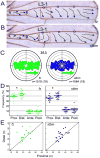
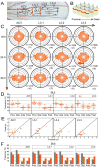
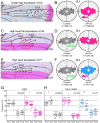
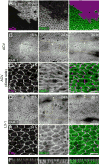
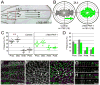
How global organ asymmetry and individual cell polarity are connected to each other is a central question in studying planar cell polarity (PCP). In the Drosophila wing, which develops PCP along its proximal-distal (P-D) axis, we previously proposed that the core PCP mediator Frizzled redistributes distally in a microtubule (MT)-dependent manner. Here, we performed organ-wide analysis of MT dynamics by introducing quantitative in vivo imaging. We observed MTs aligning along the P-D axis at the onset of redistribution and a small but significant excess of + ends-distal MTs in the proximal region of the wing. This characteristic alignment and asymmetry of MT growth was controlled by atypical cadherins Dachsous (Ds) and Fat (Ft). Furthermore, the action of Ft was mediated in part by PAR-1. All these data support the idea that the active reorientation of MT growth adjusts cell polarity along the organ axis.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. - PubMed
-
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. - PubMed
-
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. - PubMed
-
- Baena-López LA, Baonza A, García-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. - PubMed
-
- Bayraktar J, Zygmunt D, Carthew RW. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J Cell Sci. 2006;119:711–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

