Sequence-dependent prion protein misfolding and neurotoxicity
- PMID: 20817727
- PMCID: PMC2978619
- DOI: 10.1074/jbc.M110.174391
Sequence-dependent prion protein misfolding and neurotoxicity
Abstract
Prion diseases are neurodegenerative disorders caused by misfolding of the normal prion protein (PrP) into a pathogenic "scrapie" conformation. To better understand the cellular and molecular mechanisms that govern the conformational changes (conversion) of PrP, we compared the dynamics of PrP from mammals susceptible (hamster and mouse) and resistant (rabbit) to prion diseases in transgenic flies. We recently showed that hamster PrP induces spongiform degeneration and accumulates into highly aggregated, scrapie-like conformers in transgenic flies. We show now that rabbit PrP does not induce spongiform degeneration and does not convert into scrapie-like conformers. Surprisingly, mouse PrP induces weak neurodegeneration and accumulates small amounts of scrapie-like conformers. Thus, the expression of three highly conserved mammalian prion proteins in transgenic flies uncovered prominent differences in their conformational dynamics. How these properties are encoded in the amino acid sequence remains to be elucidated.
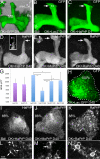
Figures







References
-
- Aguzzi A., Heikenwalder M., Polymenidou M. (2007) Nat. Rev. Mol. Cell Biol. 8, 552–561 - PubMed
-
- Barlow R. M., Rennie J. C. (1976) Res. Vet. Sci. 21, 110–111 - PubMed
-
- Gibbs C. J., Jr., Gajdusek D. C. (1973) Science 182, 67–68 - PubMed
-
- Courageot M. P., Daude N., Nonno R., Paquet S., Di Bari M. A., Le Dur A., Chapuis J., Hill A. F., Agrimi U., Laude H., Vilette D. (2008) J. Gen. Virol. 89, 341–347 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

