Mutations in C16orf57 and normal-length telomeres unify a subset of patients with dyskeratosis congenita, poikiloderma with neutropenia and Rothmund-Thomson syndrome
- PMID: 20817924
- PMCID: PMC2957322
- DOI: 10.1093/hmg/ddq371
Mutations in C16orf57 and normal-length telomeres unify a subset of patients with dyskeratosis congenita, poikiloderma with neutropenia and Rothmund-Thomson syndrome
Abstract
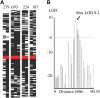
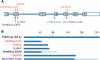
Dyskeratosis congenita (DC) is an inherited poikiloderma which in addition to the skin abnormalities is typically associated with nail dystrophy, leucoplakia, bone marrow failure, cancer predisposition and other features. Approximately 50% of DC patients remain genetically uncharacterized. All the DC genes identified to date are important in telomere maintenance. To determine the genetic basis of the remaining cases of DC, we undertook linkage analysis in 20 families and identified a common candidate gene region on chromosome 16 in a subset of these. This region included the C16orf57 gene recently identified to be mutated in poikiloderma with neutropenia (PN), an inherited poikiloderma displaying significant clinical overlap with DC. Analysis of the C16orf57 gene in our uncharacterized DC patients revealed homozygous mutations in 6 of 132 families. In addition, three of six families previously classified as Rothmund-Thomson syndrome (RTS-a poikiloderma that is sometimes confused with PN) were also found to have homozygous C16orf57 mutations. Given the role of the previous DC genes in telomere maintenance, telomere length was analysed in these patients and found to be comparable to age-matched controls. These findings suggest that mutations in C16orf57 unify a distinct set of families which clinically can be categorized as DC, PN or RTS. This study also highlights the multi-system nature (wider than just poikiloderma and neutropenia) of the clinical features of affected individuals (and therefore house-keeping function of C16orf57), a possible role for C16orf57 in apoptosis, as well as a distinct difference from previously characterized DC patients because telomere length was normal.
Figures


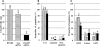
References
-
- Dokal I. Dyskeratosis congenita in all its forms. Br. J. Haematol. 2000;110:768–779. doi:10.1046/j.1365-2141.2000.02109.x. - DOI - PubMed
-
- Heiss N.S., Knight S.W., Vulliamy T.J., Klauck S.M., Wiemann S., Mason P.J., Poustka A., Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998;19:32–38. doi:10.1038/ng0598-32. - DOI - PubMed
-
- Vulliamy T., Marrone A., Goldman F., Dearlove A., Bessler M., Mason P.J., Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi:10.1038/35096585. - DOI - PubMed
-
- Armanios M., Chen J.L., Chang Y.P., Brodsky R.A., Hawkins A., Griffin C.A., Eshleman J.R., Cohen A.R., Chakravarti A., Hamosh A., et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2005;102:15960–15964. doi:10.1073/pnas.0508124102. - DOI - PMC - PubMed
-
- Walne A.J., Vulliamy T., Marrone A., Beswick R., Kirwan M., Masunari Y., Al-Qurashi F.H., Aljurf M., Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi:10.1093/hmg/ddm111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

