Ketones and lactate "fuel" tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism
- PMID: 20818174
- PMCID: PMC3047616
- DOI: 10.4161/cc.9.17.12731
Ketones and lactate "fuel" tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism
Abstract
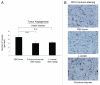
Previously, we proposed a new model for understanding the "Warburg effect" in tumor metabolism. In this scheme, cancer-associated fibroblasts undergo aerobic glycolysis and the resulting energy-rich metabolites are then transferred to epithelial cancer cells, where they enter the TCA cycle, resulting in high ATP production via oxidative phosphorylation. We have termed this new paradigm "The Reverse Warburg Effect." Here, we directly evaluate whether the end-products of aerobic glycolysis (3-hydroxy-butyrate and L-lactate) can stimulate tumor growth and metastasis, using MDA-MB-231 breast cancer xenografts as a model system. More specifically, we show that administration of 3-hydroxy-butyrate (a ketone body) increases tumor growth by ∼2.5-fold, without any measurable increases in tumor vascularization/angiogenesis. Both 3-hydroxy-butyrate and L-lactate functioned as chemo-attractants, stimulating the migration of epithelial cancer cells. Although L-lactate did not increase primary tumor growth, it stimulated the formation of lung metastases by ∼10-fold. Thus, we conclude that ketones and lactate fuel tumor growth and metastasis, providing functional evidence to support the "Reverse Warburg Effect". Moreover, we discuss the possibility that it may be unwise to use lactate-containing i.v. solutions (such as Lactated Ringer's or Hartmann's solution) in cancer patients, given the dramatic metastasis-promoting properties of L-lactate. Also, we provide evidence for the up-regulation of oxidative mitochondrial metabolism and the TCA cycle in human breast cancer cells in vivo, via an informatics analysis of the existing raw transcriptional profiles of epithelial breast cancer cells and adjacent stromal cells. Lastly, our findings may explain why diabetic patients have an increased incidence of cancer, due to increased ketone production, and a tendency towards autophagy/mitophagy in their adipose tissue.
Figures



References
-
- Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1167–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01 CA098779/CA/NCI NIH HHS/United States
- R01-CA-120876/CA/NCI NIH HHS/United States
- R01 CA120876/CA/NCI NIH HHS/United States
- R01-CA-70896/CA/NCI NIH HHS/United States
- R01-CA-098779/CA/NCI NIH HHS/United States
- R01-CA-86072/CA/NCI NIH HHS/United States
- R01-AR-055660/AR/NIAMS NIH HHS/United States
- R01-CA-080250/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01 CA107382/CA/NCI NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- P30-CA-56036/CA/NCI NIH HHS/United States
- R01-CA-107382/CA/NCI NIH HHS/United States
- R01 AR055660/AR/NIAMS NIH HHS/United States
- R01-CA-75503/CA/NCI NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
