Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats
- PMID: 20818176
- PMCID: PMC3011216
- DOI: 10.4161/mabs.2.6.13333
Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats
Abstract
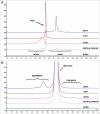
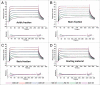
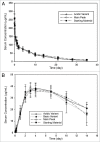
Antibody charge variants have gained considerable attention in the biotechnology industry due to their potential influence on stability and biological activity. Subtle differences in the relative proportions of charge variants are often observed during routine biomanufacture or process changes and pose a challenge to demonstrating product comparability. To gain further insights into the impact on biological activity and pharmacokinetics (PK) of monoclonal antibody (mAb) charge heterogeneity, we isolated the major charge forms of a recombinant humanized IgG1 and compared their in vitro properties and in vivo PK. The mAb starting material had a pI range of 8.7-9.1 and was composed of about 20% acidic variants, 12% basic variants, and 68% main peak. Cation exchange displacement chromatography was used to isolate the acidic, basic, and main peak fractions for animal studies. Detailed analyses were performed on the isolated fractions to identify specific chemical modification contributing to the charge differences, and were also characterized for purity and in vitro potency prior to being administered either subcutaneously (SC) or intravenously (IV) in rats. All isolated materials had similar potency and rat FcRn binding relative to the starting material. Following IV or SC administration (10 mg/kg) in rats, no difference in serum PK was observed, indicating that physiochemical modifications and pI differences among charge variants were not sufficient to result in PK changes. Thus, these results provided meaningful information for the comparative evaluation of charge-related heterogeneity of mAbs, and suggested that charge variants of IgGs do not affect the in vitro potency, FcRn binding affinity, or the PK properties in rats.
Figures
References
-
- Seiler FR, Gronski P, Kurrle R, Lüben G, Harthus HP, Ax W, et al. Monoclonal antibodies: their chemistry, functions and possible uses. Angew Chem Int Ed Engl. 1985;24:139–160.
-
- Cohen S. General structure and heterogeneity of immunoglobulins. Proc R Soc Lond B Biol Sci. 1966;166:114–123. - PubMed
-
- Boswell CA, Deng R, Lin K, Putnam WS, Lei C, Theil FP, et al. In vitro-in vivo correlation of pharmacokinetics, pharmacodynamics and metabolism for antibody therapeutics. In: Mrsny R, Daugherty A, editors. Proteins and Peptides: Pharmacokinetic, Pharmacodynamic and Metabolic Outcomes. New York: NY: Informa Healthcare; 2010. pp. 1–14.
-
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. - PubMed
-
- Carter P. Improving the efficacy of antibody-based cancer therapies. Nature Rev Cancer. 2001;1:118–129. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
